Innovative dual system approach for selective eradication of cancer cells using viral-based delivery of natural bacterial toxin-antitoxin system
- PMID: 34172933
- PMCID: PMC8342310
- DOI: 10.1038/s41388-021-01792-8
Innovative dual system approach for selective eradication of cancer cells using viral-based delivery of natural bacterial toxin-antitoxin system
Abstract
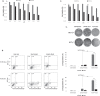
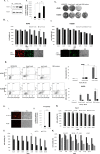
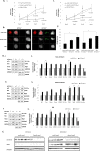
The inactivation of p53, a tumor suppressor, and the activation of the RAS oncogene are the most frequent genetic alterations in cancer. We have shown that a unique E. coli MazF-MazE toxin-antitoxin (TA) system can be used for selective and effective eradication of RAS-mutated cancer cells. This out of the box strategy holds great promise for effective cancer treatment and management. We provide proof of concept for a novel platform to selectively eradicate cancer cells using an adenoviral delivery system based on the adjusted natural bacterial system. We generated adenoviral vectors carrying the mazF toxin (pAdEasy-Py4-SV40mP-mCherry-MazF) and the antitoxin mazE (pAdEasy-RGC-SV40mP-MazE-IRES-GFP) under the regulation of RAS and p53, resp. The control vector carries the toxin without the RAS-responsive element (pAdEasy-ΔPy4-SV40mP-mCherry-MazF). In vitro, the mazF-mazE TA system (Py4-SV40mP-mCherry-MazF+RGC-SV40mP-MazE-IRES-GFP) induced massive, dose-dependent cell death, at 69% compared to 19% for the control vector, in a co-infected HCT116 cell line. In vivo, the system caused significant tumor growth inhibition of HCT116 (KRASmut/p53mut) tumors at 73 and 65% compared to PBS and ΔPY4 control groups, resp. In addition, we demonstrate 65% tumor growth inhibition in HCT116 (KRASmut/p53wt) cells, compared to the other two control groups, indicating a contribution of the antitoxin in blocking system leakage in WT RAS cells. These data provide evidence of the feasibility of using mutations in the p53 and RAS pathway to efficiently kill cancer cells. The platform, through its combination of the antitoxin (mazE) with the toxin (mazF), provides effective protection of normal cells from basal low activity or leakage of mazF.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Selective eradication of cancer cells by delivery of adenovirus-based toxins.Oncotarget. 2017 Jun 13;8(24):38581-38591. doi: 10.18632/oncotarget.16934. Oncotarget. 2017. PMID: 28445136 Free PMC article.
-
Novel Toxin-antitoxin System Xn-mazEF from Xenorhabdus nematophila: Identification, Characterization and Functional Exploration.Curr Comput Aided Drug Des. 2021;17(3):402-411. doi: 10.2174/1573409916666200625135850. Curr Comput Aided Drug Des. 2021. PMID: 32586257
-
Comparative analysis of MazEF and HicAB toxin-antitoxin systems of the cyanobacterium, Anabaena sp. PCC7120.FEMS Microbiol Lett. 2017 Jan;364(1):fnw279. doi: 10.1093/femsle/fnw279. Epub 2016 Dec 8. FEMS Microbiol Lett. 2017. PMID: 27940461
-
Diagnostic and therapeutic application of telomerase-specific oncolytic adenoviral agents.Front Biosci. 2008 Jan 1;13:1881-6. doi: 10.2741/2807. Front Biosci. 2008. PMID: 17981675 Review.
-
INGN 201: Ad-p53, Ad5CMV-p53, adenoviral p53, p53 gene therapy--introgen, RPR/INGN 201.Drugs R D. 2007;8(3):176-87. doi: 10.2165/00126839-200708030-00005. Drugs R D. 2007. PMID: 17472413 Review.
Cited by
-
Lipid nanoparticles-loaded with toxin mRNA represents a new strategy for the treatment of solid tumors.Theranostics. 2023 Jun 12;13(11):3497-3508. doi: 10.7150/thno.82228. eCollection 2023. Theranostics. 2023. PMID: 37441597 Free PMC article.
-
Applications of toxin-antitoxin systems in synthetic biology.Eng Microbiol. 2023 Jan 18;3(2):100069. doi: 10.1016/j.engmic.2023.100069. eCollection 2023 Jun. Eng Microbiol. 2023. PMID: 39629251 Free PMC article. Review.
-
Environmental insults and compensative responses: when microbiome meets cancer.Discov Oncol. 2023 Jul 15;14(1):130. doi: 10.1007/s12672-023-00745-9. Discov Oncol. 2023. PMID: 37453005 Free PMC article. Review.
-
Bacterial Toxin-Antitoxin Systems' Cross-Interactions-Implications for Practical Use in Medicine and Biotechnology.Toxins (Basel). 2023 Jun 4;15(6):380. doi: 10.3390/toxins15060380. Toxins (Basel). 2023. PMID: 37368681 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

