Intravitreal injections: past trends and future projections within a UK tertiary hospital
- PMID: 34172943
- PMCID: PMC8227364
- DOI: 10.1038/s41433-021-01646-3
Intravitreal injections: past trends and future projections within a UK tertiary hospital
Abstract
Aims: To describe past trends and future projections for the number of intravitreal injections being administered at a large tertiary hospital in London, United Kingdom.
Methods: Retrospective data from Moorfields Eye Hospital were collected using the electronic medical record system. Descriptive statistics were used to visualise overall trends. Time series forecasting was used to predict the number of injections that will be administered up to and including the year 2029.
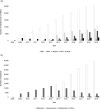
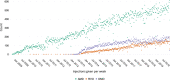
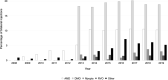
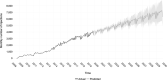
Results: The number of injections has increased nearly 11-fold from 2009 to 2019, with a total of 44,924 injections delivered in 2019. The majority of injections were given for the treatment of neovascular age-related macular degeneration. Aflibercept formed 87% of injections administered in 2019. The number of injections is predicted to continue to increase every year, with nearly 83,000 injections forecasted in the year 2029.
Conclusion: The demand for intravitreal injections has increased substantially over the last decade and is predicted to further increase. Healthcare systems will need to adapt to accommodate the high demand. Other solutions may include longer-acting therapies to reduce the treatment burden.
© 2021. The Author(s).
Conflict of interest statement
Independent of this work, RC is an employee of Google LLC and owns Alphabet stock. PJP has acted as a consultant for Bayer UK, Novartis UK and Roche UK. Dr. Balaskas has acted as a consultant for Roche and Novartis and has received speaker fees from Novartis, Bayer, Allergan, Alimera, Topcon and Heidelberg Engineering. RDH has acted as a consultant for Bayer, Novartis, Allergan, Alimera and Roche, and has received speaker fees from Ellex, Novartis, Alimera, Roche, Allergan, and Bayer. PAK has acted as a consultant for DeepMind, Roche, Novartis, and Apellis and is an equity owner in Big Picture Medical. He has received speaker fees from Heidelberg Engineering, Topcon, Allergan, and Bayer.
Figures

References
-
- National Institute for Health and Care Excellence. Age-related macular degeneration. 2018. Available at: https://www.nice.org.uk/guidance/ng82/ [Accessed April 30, 2020]. - PubMed
-
- European Medicines Agency. Lucentis. 2018. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/lucentis [Accessed November 8, 2020].
-
- National Institute for Health and Care Excellence (NICE). Ranibizumab and pegaptanib for the treatment of age-related macular degeneration. 2008.
-
- Center for drug evaluation and research. BLA application number: 125156. Approval Letter. 2006. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/125156s0000_Luce....
-
- Center for drug evaluation and research. BLA application: 125387Orig1s000. Approval Letter. 2011. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/125387Orig1s000A....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

