COVID-19 convalescent plasma cohort study: Evaluation of the association between both donor and recipient neutralizing antibody titers and patient outcomes
- PMID: 34173248
- PMCID: PMC8447313
- DOI: 10.1111/trf.16573
COVID-19 convalescent plasma cohort study: Evaluation of the association between both donor and recipient neutralizing antibody titers and patient outcomes
Abstract
Background: Current evidence regarding COVID-19 convalescent plasma (CCP) transfusion practices is limited and heterogeneous. We aimed to determine the impact of the use of CCP transfusion in patients with previous circulating neutralizing antibodies (nAbs) in COVID-19.
Methods: Prospective cohort including 102 patients with COVID-19 transfused with ABO compatible CCP on days 0-2 after enrollment. Clinical status of patients was assessed using the adapted World Health Organization (WHO) ordinal scale on days 0, 5, and 14. The nAbs titration was performed using the cytopathic effect-based virus neutralization test with SARS-CoV-2 (GenBank MT126808.1). The primary outcome was clinical improvement on day 14, defined as a reduction of at least two points on the adapted WHO ordinal scale. Secondary outcomes were the number of intensive care unit (ICU)-free days and the number of invasive mechanical ventilation-free days.
Results: Both nAbs of CCP units transfused (p < 0.001) and nAbs of patients before CCP transfusions (p = 0.028) were associated with clinical improvements by day 14. No significant associations between nAbs of patients or CCP units transfused were observed in the number of ICU or mechanical ventilation-free days. Administration of CCP units after 10 days of symptom onset resulted in a decrease in ICU-free days (p < 0.001) and mechanical ventilation-free days (p < 0.001).
Conclusion: Transfusion of high titer nAbs CCP units may be a determinant in clinical strategies against COVID-19. We consider these data as useful parameters to guide future CCP transfusion practices.
Keywords: COVID-19; SARS-CoV-2; convalescent plasma transfusion; neutralizing antibodies.
© 2021 AABB.
Conflict of interest statement
The authors have disclosed no conflicts of interest.
Figures
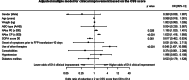
Similar articles
-
Use of Outpatient-Derived COVID-19 Convalescent Plasma in COVID-19 Patients Before Seroconversion.Front Immunol. 2021 Sep 14;12:739037. doi: 10.3389/fimmu.2021.739037. eCollection 2021. Front Immunol. 2021. PMID: 34594341 Free PMC article.
-
Screening for SARS-CoV-2 antibodies in convalescent plasma in Brazil: Preliminary lessons from a voluntary convalescent donor program.Transfusion. 2020 Dec;60(12):2938-2951. doi: 10.1111/trf.16065. Epub 2020 Sep 16. Transfusion. 2020. PMID: 32935877 Free PMC article.
-
Outpatient COVID-19 convalescent plasma recipient antibody thresholds correlated to reduced hospitalizations within a randomized trial.JCI Insight. 2024 Mar 14;9(8):e178460. doi: 10.1172/jci.insight.178460. JCI Insight. 2024. PMID: 38483534 Free PMC article. Clinical Trial.
-
COVID 19 convalescent plasma: Is there still a place for CCP?Transfus Apher Sci. 2023 Apr;62(2):103680. doi: 10.1016/j.transci.2023.103680. Epub 2023 Feb 24. Transfus Apher Sci. 2023. PMID: 36870907 Free PMC article. Review.
-
Clinical predictors of SARS-CoV-2 neutralizing antibody titers in COVID-19 convalescents: Implications for convalescent plasma donor recruitment.Eur J Haematol. 2021 Jul;107(1):24-28. doi: 10.1111/ejh.13630. Epub 2021 Apr 20. Eur J Haematol. 2021. PMID: 33780551 Free PMC article. Review.
Cited by
-
Evaluation of a COVID-19 convalescent plasma program at a U.S. academic medical center.PLoS One. 2022 Dec 8;17(12):e0277707. doi: 10.1371/journal.pone.0277707. eCollection 2022. PLoS One. 2022. PMID: 36480499 Free PMC article. Clinical Trial.
-
Use of convalescent plasma in COVID-19 treatment: is clinical severity more important than the intervention?Einstein (Sao Paulo). 2024 Dec 16;22:eAO0563. doi: 10.31744/einstein_journal/2024AO0563. eCollection 2024. Einstein (Sao Paulo). 2024. PMID: 39699400 Free PMC article.
-
Dynamics of Cytokine, SARS-CoV-2-Specific IgG, and Neutralizing Antibody Levels in COVID-19 Patients Treated with Convalescent Plasma.Diseases. 2023 Aug 30;11(3):112. doi: 10.3390/diseases11030112. Diseases. 2023. PMID: 37754308 Free PMC article.
-
Review of therapeutic mechanisms and applications based on SARS-CoV-2 neutralizing antibodies.Front Microbiol. 2023 Mar 16;14:1122868. doi: 10.3389/fmicb.2023.1122868. eCollection 2023. Front Microbiol. 2023. PMID: 37007494 Free PMC article. Review.
-
High-Dose Convalescent Plasma for Treatment of Severe COVID-19.Emerg Infect Dis. 2022 Mar;28(3):548-555. doi: 10.3201/eid2803.212299. Epub 2022 Jan 26. Emerg Infect Dis. 2022. PMID: 35081022 Free PMC article. Clinical Trial.
References
-
- Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: a randomized clinical trial. JAMA. 2020;324:460–470. 10.1001/jama.2020.10044 Erratum in: JAMA. 2020;324(5):519. PMID: 32492084; PMCID: PMC7270883. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

