Oligonucleotide Phosphorothioates Enter Cells by Thiol-Mediated Uptake
- PMID: 34173696
- PMCID: PMC8456962
- DOI: 10.1002/anie.202107327
Oligonucleotide Phosphorothioates Enter Cells by Thiol-Mediated Uptake
Abstract
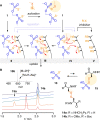
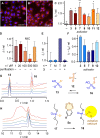
Oligonucleotide phosphorothioates (OPS) are DNA or RNA mimics where one phosphate oxygen is replaced by a sulfur atom. They have been shown to enter mammalian cells much more efficiently than non-modified DNA. Thus, solving one of the key challenges with oligonucleotide technology, OPS became very useful in practice, with several FDA-approved drugs on the market or in late clinical trials. However, the mechanism accounting for this facile cellular uptake is unknown. Here, we show that OPS enter cells by thiol-mediated uptake. The transient adaptive network produced by dynamic covalent pseudo-disulfide exchange is characterized in action. Inhibitors with nanomolar efficiency are provided, together with activators that reduce endosomal capture for efficient delivery of OPS into the cytosol, the site of action.
Keywords: cellular uptake; dynamic covalent chemistry; oligonucleotides; phosphorothioates.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Ring Tension Applied to Thiol-Mediated Cellular Uptake.Angew Chem Int Ed Engl. 2015 Jun 15;54(25):7328-31. doi: 10.1002/anie.201502358. Epub 2015 Apr 29. Angew Chem Int Ed Engl. 2015. PMID: 25925500
-
Synthesis and cellular uptake of fluorescently labeled multivalent hyaluronan disaccharide conjugates of oligonucleotide phosphorothioates.Bioconjug Chem. 2008 Dec;19(12):2549-58. doi: 10.1021/bc800260y. Bioconjug Chem. 2008. PMID: 19053300
-
Receptor-Mediated Uptake of Phosphorothioate Antisense Oligonucleotides in Different Cell Types of the Liver.Nucleic Acid Ther. 2018 Jun;28(3):119-127. doi: 10.1089/nat.2017.0709. Epub 2018 Feb 9. Nucleic Acid Ther. 2018. PMID: 29425080 Free PMC article. Review.
-
Phosphorothioates, essential components of therapeutic oligonucleotides.Nucleic Acid Ther. 2014 Dec;24(6):374-87. doi: 10.1089/nat.2014.0506. Nucleic Acid Ther. 2014. PMID: 25353652 Review.
-
Cellular uptake mediated by epidermal growth factor receptor facilitates the intracellular activity of phosphorothioate-modified antisense oligonucleotides.Nucleic Acids Res. 2018 Apr 20;46(7):3579-3594. doi: 10.1093/nar/gky145. Nucleic Acids Res. 2018. PMID: 29514240 Free PMC article.
Cited by
-
Cyclic Thiosulfonates for Thiol-Mediated Uptake: Cascade Exchangers, Transporters, Inhibitors.JACS Au. 2022 Mar 22;2(4):839-852. doi: 10.1021/jacsau.1c00573. eCollection 2022 Apr 25. JACS Au. 2022. PMID: 35557769 Free PMC article.
-
IMT504 protects beta cells against apoptosis and maintains beta cell identity, without modifying proliferation.Physiol Rep. 2023 Aug;11(15):e15790. doi: 10.14814/phy2.15790. Physiol Rep. 2023. PMID: 37568265 Free PMC article.
-
Therapeutic Oligonucleotides: An Outlook on Chemical Strategies to Improve Endosomal Trafficking.Cells. 2023 Sep 11;12(18):2253. doi: 10.3390/cells12182253. Cells. 2023. PMID: 37759475 Free PMC article. Review.
-
Multimeric Conjugates Using Engineered Peptide Scaffolds for Efficient siRNA Delivery.Bioconjug Chem. 2025 Jun 18;36(6):1299-1310. doi: 10.1021/acs.bioconjchem.5c00156. Epub 2025 May 29. Bioconjug Chem. 2025. PMID: 40443118 Free PMC article.
-
Synthesis of Seven Indolizine-Derived Pentathiepines: Strong Electronic Structure Response to Nitro Substitution in Position C-9.Molecules. 2023 Dec 30;29(1):216. doi: 10.3390/molecules29010216. Molecules. 2023. PMID: 38202800 Free PMC article.
References
-
- None
-
- Zhou J., Sun L., Wang L., Liu Y., Li J., Li J., Li J., Yang H., Angew. Chem. Int. Ed. 2019, 58, 5236–5240; - PubMed
- Angew. Chem. 2019, 131, 5290–5294;
-
- Zhou J., Shao Z., Liu J., Duan Q., Wang X., Li J., Yang H., ACS Appl. Bio Mater. 2020, 3, 2686–2701; - PubMed
-
- Kohata A., Hashim P. K., Okuro K., Aida T., J. Am. Chem. Soc. 2019, 141, 2862–2866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

