Human coronary microvascular contractile dysfunction associates with viable synthetic smooth muscle cells
- PMID: 34173824
- PMCID: PMC9239576
- DOI: 10.1093/cvr/cvab218
Human coronary microvascular contractile dysfunction associates with viable synthetic smooth muscle cells
Abstract
Aims: Coronary microvascular smooth muscle cells (SMCs) respond to luminal pressure by developing myogenic tone (MT), a process integral to the regulation of microvascular perfusion. The cellular mechanisms underlying poor myogenic reactivity in patients with heart valve disease are unknown and form the focus of this study.
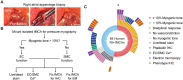
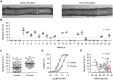
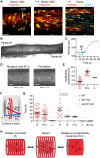
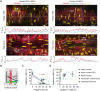
Methods and results: Intramyocardial coronary micro-arteries (IMCAs) isolated from human and pig right atrial (RA) appendage and left ventricular (LV) biopsies were studied using pressure myography combined with confocal microscopy. All RA- and LV-IMCAs from organ donors and pigs developed circa 25% MT. In contrast, 44% of human RA-IMCAs from 88 patients with heart valve disease had poor (<10%) MT yet retained cell viability and an ability to raise cytoplasmic Ca2+ in response to vasoconstrictor agents. Comparing across human heart chambers and species, we found that based on patient medical history and six tests, the strongest predictor of poor MT in IMCAs was increased expression of the synthetic marker caldesmon relative to the contractile marker SM-myosin heavy chain. In addition, high resolution imaging revealed a distinct layer of longitudinally aligned SMCs between ECs and radial SMCs, and we show poor MT was associated with disruptions in these cellular alignments.
Conclusion: These data demonstrate the first use of atrial and ventricular biopsies from patients and pigs to reveal that impaired coronary MT reflects a switch of viable SMCs towards a synthetic phenotype, rather than a loss of SMC viability. These arteries represent a model for further studies of coronary microvascular contractile dysfunction.
Keywords: Ca2+; Coronary arterioles; Coronary microvascular function; Heart valve disease; Human; Microvascular perfusion; Myogenic tone; Smooth muscle cell; Synthetic phenotype; Ultrastructure; signalling.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures




Similar articles
-
Signaling and structures underpinning conducted vasodilation in human and porcine intramyocardial coronary arteries.Front Cardiovasc Med. 2022 Aug 12;9:980628. doi: 10.3389/fcvm.2022.980628. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36035957 Free PMC article.
-
Neutralization of S100A4 induces stabilization of atherosclerotic plaques: role of smooth muscle cells.Cardiovasc Res. 2022 Jan 7;118(1):141-155. doi: 10.1093/cvr/cvaa311. Cardiovasc Res. 2022. PMID: 33135065 Free PMC article.
-
Intimal smooth muscle cells of porcine and human coronary artery express S100A4, a marker of the rhomboid phenotype in vitro.Circ Res. 2007 Apr 13;100(7):1055-62. doi: 10.1161/01.RES.0000262654.84810.6c. Epub 2007 Mar 8. Circ Res. 2007. PMID: 17347479
-
Dynamic and diverse changes in the functional properties of vascular smooth muscle cells in pulmonary hypertension.Cardiovasc Res. 2018 Mar 15;114(4):551-564. doi: 10.1093/cvr/cvy004. Cardiovasc Res. 2018. PMID: 29385432 Free PMC article. Review.
-
Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles.Compr Physiol. 2017 Mar 16;7(2):485-581. doi: 10.1002/cphy.c160011. Compr Physiol. 2017. PMID: 28333380 Free PMC article. Review.
Cited by
-
Tracking endothelium-dependent NO release in pressurized arteries.Front Physiol. 2023 Jan 24;14:1108943. doi: 10.3389/fphys.2023.1108943. eCollection 2023. Front Physiol. 2023. PMID: 36760530 Free PMC article.
-
Global trends and frontiers in research on coronary microvascular dysfunction: a bibliometric analysis from 2002 to 2022.Eur J Med Res. 2022 Nov 5;27(1):233. doi: 10.1186/s40001-022-00869-8. Eur J Med Res. 2022. PMID: 36335406 Free PMC article. Review.
-
Downregulation of the CD151 protects the cardiac function by the crosstalk between the endothelial cells and cardiomyocytes via exosomes.PLoS One. 2024 Feb 13;19(2):e0297121. doi: 10.1371/journal.pone.0297121. eCollection 2024. PLoS One. 2024. PMID: 38349935 Free PMC article.
-
Signaling and structures underpinning conducted vasodilation in human and porcine intramyocardial coronary arteries.Front Cardiovasc Med. 2022 Aug 12;9:980628. doi: 10.3389/fcvm.2022.980628. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36035957 Free PMC article.
-
Opportunities and challenges for the use of human samples in translational cardiovascular research: a scientific statement of the ESC Working Group on Cellular Biology of the Heart, the ESC Working Group on Cardiovascular Surgery, the ESC Council on Basic Cardiovascular Science, the ESC Scientists of Tomorrow, the European Association of Percutaneous Cardiovascular Interventions of the ESC, and the Heart Failure Association of the ESC.Cardiovasc Res. 2025 May 23;121(5):702-729. doi: 10.1093/cvr/cvaf023. Cardiovasc Res. 2025. PMID: 40084813 Review.
References
-
- Marcus ML, Chilian WM, Kanatsuka H, Dellsperger KC, Eastham CL, Lamping KG.. Understanding the coronary circulation through studies at the microvascular level. Circulation 1990;82:1–7. - PubMed
-
- Kuo L, Chilian WM, Davis MJ.. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol Heart Circ Physiol 1991;261:H1706–H1715. - PubMed
-
- Muller JM, Davis MJ, Chilian WM.. Integrated regulation of pressure and flow in the coronary microcirculation. Cardiovasc Res 1996;32:668–678. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

