Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study
- PMID: 34174190
- PMCID: PMC8221734
- DOI: 10.1016/S1473-3099(21)00330-3
Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study
Erratum in
-
Correction to Lancet Infect Dis 2021; published online June 23. https://doi.org/10.1016/ S1473-3099(21)00330-3.Lancet Infect Dis. 2021 Aug;21(8):e208. doi: 10.1016/S1473-3099(21)00397-2. Epub 2021 Jul 5. Lancet Infect Dis. 2021. PMID: 34237259 Free PMC article. No abstract available.
Abstract
Background: On Dec 8, 2020, deployment of the first SARS-CoV-2 vaccination authorised for UK use (BNT162b2 mRNA vaccine) began, followed by an adenoviral vector vaccine ChAdOx1 nCoV-19 on Jan 4, 2021. Care home residents and staff, frontline health-care workers, and adults aged 80 years and older were vaccinated first. However, few data exist regarding the effectiveness of these vaccines in older people with many comorbidities. In this post-implementation evaluation of two COVID-19 vaccines, we aimed to determine the effectiveness of one dose in reducing COVID-19-related admissions to hospital in people of advanced age.
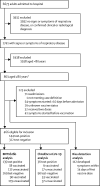
Methods: This prospective test-negative case-control study included adults aged at least 80 years who were admitted to hospital in two NHS trusts in Bristol, UK with signs and symptoms of respiratory disease. Patients who developed symptoms before receiving their vaccine or those who received their vaccine after admission to hospital were excluded, as were those with symptoms that started more than 10 days before hospital admission. We did logistic regression analysis, controlling for time (week), sex, index of multiple deprivations, and care residency status, and sensitivity analyses matched for time and sex using a conditional logistic model adjusting for index of multiple deprivations and care residency status. This study is registered with ISRCTN, number 39557.
Findings: Between Dec 18, 2020, and Feb 26, 2021, 466 adults were eligible (144 test-positive and 322 test-negative). 18 (13%) of 135 people with SARS-CoV-2 infection and 90 (34%) of 269 controls received one dose of BNT162b2. The adjusted vaccine effectiveness was 71·4% (95% CI 46·5-90·6). Nine (25%) of 36 people with COVID-19 infection and 53 (59%) of 90 controls received one dose of ChAdOx1 nCoV-19. The adjusted vaccine effectiveness was 80·4% (95% CI 36·4-94·5). When BNT162b2 effectiveness analysis was restricted to the period covered by ChAdOx1 nCoV-19, the estimate was 79·3% (95% CI 47·0-92·5).
Interpretation: One dose of either BNT162b2 or ChAdOx1 nCoV-19 resulted in substantial risk reductions of COVID-19-related hospitalisation in people aged at least 80 years.
Funding: Pfizer.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests CH is principal investigator of the Avon CAP study which is an investigator-led University of Bristol study funded by Pfizer and has previously received support from the NIHR in an academic clinical fellowship. JO is a co-investigator on the Avon CAP study. LD is further supported by UKRI through the JUNIPER consortium (grant number MR/V038613/1), MRC (grant number MC/PC/19067), EPSRC (EP/V051555/1 and The Alan Turing Institute, grant EP/N510129/1). AF is a member of the JCVI and is chair of WHO's European Technical Advisory Group of Experts on Immunization committee. In addition to receiving funding from Pfizer as chief investigator of this study, he leads another project investigating transmission of respiratory bacteria in families jointly funded by Pfizer and the Gates Foundation and is an investigator in trials of COVID-19 vaccines including ChAdOx1 nCOV-19, Janssen, and Valneva vaccines. The other authors have no relevant conflicts of interest to declare.
Figures

Comment in
-
Single-dose SARS-CoV-2 vaccination efficacy in the elderly.Lancet Infect Dis. 2021 Nov;21(11):1474-1475. doi: 10.1016/S1473-3099(21)00354-6. Epub 2021 Jun 23. Lancet Infect Dis. 2021. PMID: 34174192 Free PMC article. No abstract available.
References
-
- WHO WHO coronavirus disease (COVID-19) dashboard. Feb 24, 2021. https://covid19.who.int/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

