Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study
- PMID: 34174193
- PMCID: PMC8221738
- DOI: 10.1016/S1473-3099(21)00289-9
Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study
Abstract
Background: The effectiveness of SARS-CoV-2 vaccines in older adults living in long-term care facilities is uncertain. We investigated the protective effect of the first dose of the Oxford-AstraZeneca non-replicating viral-vectored vaccine (ChAdOx1 nCoV-19; AZD1222) and the Pfizer-BioNTech mRNA-based vaccine (BNT162b2) in residents of long-term care facilities in terms of PCR-confirmed SARS-CoV-2 infection over time since vaccination.
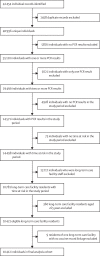
Methods: The VIVALDI study is a prospective cohort study that commenced recruitment on June 11, 2020, to investigate SARS-CoV-2 transmission, infection outcomes, and immunity in residents and staff in long-term care facilities in England that provide residential or nursing care for adults aged 65 years and older. In this cohort study, we included long-term care facility residents undergoing routine asymptomatic SARS-CoV-2 testing between Dec 8, 2020 (the date the vaccine was first deployed in a long-term care facility), and March 15, 2021, using national testing data linked within the COVID-19 Datastore. Using Cox proportional hazards regression, we estimated the relative hazard of PCR-positive infection at 0-6 days, 7-13 days, 14-20 days, 21-27 days, 28-34 days, 35-48 days, and 49 days and beyond after vaccination, comparing unvaccinated and vaccinated person-time from the same cohort of residents, adjusting for age, sex, previous infection, local SARS-CoV-2 incidence, long-term care facility bed capacity, and clustering by long-term care facility. We also compared mean PCR cycle threshold (Ct) values for positive swabs obtained before and after vaccination. The study is registered with ISRCTN, number 14447421.
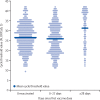
Findings: 10 412 care home residents aged 65 years and older from 310 LTCFs were included in this analysis. The median participant age was 86 years (IQR 80-91), 7247 (69·6%) of 10 412 residents were female, and 1155 residents (11·1%) had evidence of previous SARS-CoV-2 infection. 9160 (88·0%) residents received at least one vaccine dose, of whom 6138 (67·0%) received ChAdOx1 and 3022 (33·0%) received BNT162b2. Between Dec 8, 2020, and March 15, 2021, there were 36 352 PCR results in 670 628 person-days, and 1335 PCR-positive infections (713 in unvaccinated residents and 612 in vaccinated residents) were included. Adjusted hazard ratios (HRs) for PCR-positive infection relative to unvaccinated residents declined from 28 days after the first vaccine dose to 0·44 (95% CI 0·24-0·81) at 28-34 days and 0·38 (0·19-0·77) at 35-48 days. Similar effect sizes were seen for ChAdOx1 (adjusted HR 0·32, 95% CI 0·15-0·66) and BNT162b2 (0·35, 0·17-0·71) vaccines at 35-48 days. Mean PCR Ct values were higher for infections that occurred at least 28 days after vaccination than for those occurring before vaccination (31·3 [SD 8·7] in 107 PCR-positive tests vs 26·6 [6·6] in 552 PCR-positive tests; p<0·0001).
Interpretation: Single-dose vaccination with BNT162b2 and ChAdOx1 vaccines provides substantial protection against infection in older adults from 4-7 weeks after vaccination and might reduce SARS-CoV-2 transmission. However, the risk of infection is not eliminated, highlighting the ongoing need for non-pharmaceutical interventions to prevent transmission in long-term care facilities.
Funding: UK Government Department of Health and Social Care.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests LS reports grants from the UK Department of Health and Social Care during the conduct of the study and is a member of the Social Care Working Group, which reports to the Scientific Advisory Group for Emergencies. AH is a member of the New and Emerging Respiratory Virus Threats Advisory Group at the UK Department of Health. DD is an employee of Palantir Technologies UK, which provided the data platform that was used for this study under a general contract with the UK Government (DHSC/NHS England and Improvement). All other authors declare no competing interests.
Figures
Comment in
-
Single-dose SARS-CoV-2 vaccination efficacy in the elderly.Lancet Infect Dis. 2021 Nov;21(11):1474-1475. doi: 10.1016/S1473-3099(21)00354-6. Epub 2021 Jun 23. Lancet Infect Dis. 2021. PMID: 34174192 Free PMC article. No abstract available.
-
Hospitalisation among vaccine breakthrough COVID-19 infections.Lancet Infect Dis. 2021 Nov;21(11):1485-1486. doi: 10.1016/S1473-3099(21)00558-2. Epub 2021 Sep 7. Lancet Infect Dis. 2021. PMID: 34506735 Free PMC article. No abstract available.
References
-
- International Long-Term Care Policy Network International reports on COVID-19 and long-term care. https://ltccovid.org/international-reports-on-covid-19-and-long-term-care
-
- GOV.UK
- Independent report—priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI. 30 December 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavir...
-
- GOV.UK
- MHRA guidance on coronavirus (COVID-19) March 19, 2020. https://www.gov.uk/government/collections/mhra-guidance-on-coronavirus-c...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous