In vivo targeted delivery of nucleic acids and CRISPR genome editors enabled by GSH-responsive silica nanoparticles
- PMID: 34174352
- PMCID: PMC8383466
- DOI: 10.1016/j.jconrel.2021.06.030
In vivo targeted delivery of nucleic acids and CRISPR genome editors enabled by GSH-responsive silica nanoparticles
Abstract
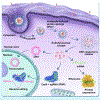
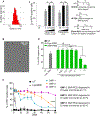
The rapid development of gene therapy and genome editing techniques brings up an urgent need to develop safe and efficient nanoplatforms for nucleic acids and CRISPR genome editors. Herein we report a stimulus-responsive silica nanoparticle (SNP) capable of encapsulating biomacromolecules in their active forms with a high loading content and loading efficiency as well as a well-controlled nanoparticle size (~50 nm). A disulfide crosslinker was integrated into the silica network, endowing SNP with glutathione (GSH)-responsive cargo release capability when internalized by target cells. An imidazole-containing component was incorporated into the SNP to enhance the endosomal escape capability. The SNP can deliver various cargos, including nucleic acids (e.g., DNA and mRNA) and CRISPR genome editors (e.g., Cas9/sgRNA ribonucleoprotein (RNP), and RNP with donor DNA) with excellent efficiency and biocompatibility. The SNP surface can be PEGylated and functionalized with different targeting ligands. In vivo studies showed that subretinally injected SNP conjugated with all-trans-retinoic acid (ATRA) and intravenously injected SNP conjugated with GalNAc can effectively deliver mRNA and RNP to murine retinal pigment epithelium (RPE) cells and liver cells, respectively, leading to efficient genome editing. Overall, the SNP is a promising nanoplatform for various applications including gene therapy and genome editing.
Keywords: CRISPR-Cas9 genome editing; Gene delivery; Silica nanoparticle.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures





References
-
- Zhang H, Lee M-Y, Hogg MG, Dordick JS, Sharfstein ST, Gene delivery in three-dimensional cell cultures by superparamagnetic nanoparticles, ACS Nano 4 (2010) 4733–4743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

