Humoral and Cellular Responses to mRNA-1273 and BNT162b2 SARS-CoV-2 Vaccines Administered to Hemodialysis Patients
- PMID: 34174364
- PMCID: PMC8223037
- DOI: 10.1053/j.ajkd.2021.06.002
Humoral and Cellular Responses to mRNA-1273 and BNT162b2 SARS-CoV-2 Vaccines Administered to Hemodialysis Patients
Abstract
Rationale & objective: Patients with kidney failure who are receiving maintenance dialysis have a higher risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and worse clinical outcomes after coronavirus disease 2019 (COVID-19) than the general population. Therefore, immunization against SARS-CoV-2 with effective vaccines is an important component of health-maintenance strategies for these patients. This study evaluated the humoral and cellular responses to messenger RNA (mRNA) SARS-CoV-2 vaccines in this population.
Study design: Observational prospective multicenter cohort study.
Setting & participants: 205 patients treated at 3 dialysis units at the Hospital Clínic of Barcelona (Spain) were vaccinated from February 3 to April 4, 2021, and followed until April 23, 2021.
Exposure: Immunization with either the mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 mRNA vaccine.
Outcome: Seroconversion, defined as the detection of IgG antibodies to the receptor-binding domain of the S1 spike antigen of SARS-CoV-2 (anti-S1-RBD IgG), and the identification of activated CD4+T cells 3 weeks after completing vaccination. Anti-S1-RBD IgG levels were also analyzed as a secondary outcome.
Analytical approach: Univariate and multivariable logistic and multiple linear regression models were used to evaluate the associations between vaccination and study outcomes.
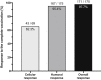
Results: We found that 97.7% of 175 vaccinated patients who were seronegative at baseline developed a response (humoral, cellular, or both); 95.4% of these patients seroconverted, while 62% of those tested for cellular immunity had a positive response. Greater age and immunosuppressive treatment were associated with lower antibody levels.
Limitations: Mandatory vaccine administration by health authorities. Anti-S1-RBD IgG levels were reported up to 150U/mL and cellular immune responses were characterized qualitatively. Antibody assay and cellular response assessment may not be comparable with previously published laboratory approaches.
Conclusions: Immunization with mRNA vaccines generated a humoral and cellular immune response in a high proportion of patients with kidney failure receiving maintenance dialysis. These findings as well as the high risk of infection and poor clinical outcomes among these patients make their vaccination a health priority.
Keywords: Antibody response; COVID-19; COVID-19 vaccines; SARS-CoV-2; cellular response; end-stage renal disease (ESRD); hemodialysis; humoral response; immunogenicity; immunosuppression; mRNA vaccines; seroconversion.
Copyright © 2021 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

