Efficacy and safety of ReDuNing injection as a treatment for COVID-19 and its inhibitory effect against SARS-CoV-2
- PMID: 34174375
- PMCID: PMC8223030
- DOI: 10.1016/j.jep.2021.114367
Efficacy and safety of ReDuNing injection as a treatment for COVID-19 and its inhibitory effect against SARS-CoV-2
Abstract
Background: Although the rapid emergence of coronavirus disease 2019 (COVID-19) poses a considerable threat to global public health, no specific treatment is available for COVID-19. ReDuNing injection (RDN) is a traditional Chinese medicine known to exert antibacterial, antiviral, antipyretic, and anti-inflammatory effects. In addition, RDN has been recommended in the diagnosis and treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-associated pneumonia by the National Health Council and the National Administration of Chinese Medicine. However, there is no information regarding its efficacy against COVID-19.
Aim of study: This study was designed to determine the clinical efficacy of RDN in patients with COVID-19 and characterize its antiviral activity against SARS-CoV-2 in vitro.
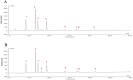
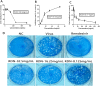
Materials and methods: A total of 50 adults with COVID-19 were included in this study, and the primary endpoint was recovery from clinical symptoms following 14 days of treatment. General improvements were defined as the disappearance of the major symptoms of infection including fever, fatigue, and cough. The secondary endpoints included the proportion of patients who achieved clinical symptom amelioration on days 7 and 10, time to clinical recovery, time to a negative nucleic acid test result, duration of hospitalization, and time to defervescence. Plaque reduction and cytopathic effect assays were also performed in vitro, and reverse-transcription quantitative PCR was performed to evaluate the expression of inflammatory cytokines (TNF-α, IP-10, MCP-1, IL-6, IFN-α, IFN-γ, IL-2 and CCL-5) during SARS-CoV-2 infection.
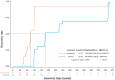
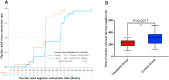
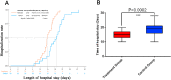
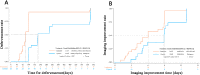
Results: The RDN group exhibited a shorter median time for the resolution of clinical symptoms (120 vs. 220 h, P < 0.0001), less time to a negative PCR test result (215 vs. 310 h, P = 0.0017), shorter hospitalization (14.8 vs. 18.5 days, P = 0.0002), and lower timeframe for defervescence (24.5 vs. 75 h, P = 0.0001) than the control group. In addition, time to improved imaging was also shorter in the RDN group than in the control group (6 vs.8.9 days, P = 0.0273); symptom resolution rates were higher in the RDN group than in the control group at 7 (96.30% vs. 39.13%, P < 0.0001) and 10 days (96.30% vs. 56.52%, P = 0.0008). No allergic reactions or anaphylactic responses were reported in this trial. RDN markedly inhibited SARS-CoV-2 proliferation and viral plaque formation in vitro. In addition, RDN significantly reduced inflammatory cytokine production in infected cells.
Conclusions: RDN relieves clinical symptoms in patients with COVID-19 and reduces SARS-CoV-2 infection by regulating inflammatory cytokine-related disorders, suggestion that this medication might be a safe and effective treatment for COVID-19.
Keywords: COVID-19; Inflammatory cytokines; ReDuNing injection; SARS-CoV-2; Traditional Chinese medicine.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Efficacy and safety of Reduning injection in the treatment of COVID-19: a randomized, multicenter clinical study.Ann Palliat Med. 2021 May;10(5):5146-5155. doi: 10.21037/apm-20-2121. Epub 2021 Apr 9. Ann Palliat Med. 2021. PMID: 33894725 Clinical Trial.
-
Efficacy of Traditional Chinese Medicine, Maxingshigan-Weijing in the management of COVID-19 patients with severe acute respiratory syndrome: A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Dec 23;21(1):1029. doi: 10.1186/s13063-020-04970-3. Trials. 2020. PMID: 33357239 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Chinese herbal medicine: Fighting SARS-CoV-2 infection on all fronts.J Ethnopharmacol. 2021 Apr 24;270:113869. doi: 10.1016/j.jep.2021.113869. Epub 2021 Jan 21. J Ethnopharmacol. 2021. PMID: 33485973 Free PMC article.
-
Interferon therapy in patients with SARS, MERS, and COVID-19: A systematic review and meta-analysis of clinical studies.Eur J Pharmacol. 2021 Sep 5;906:174248. doi: 10.1016/j.ejphar.2021.174248. Epub 2021 Jun 12. Eur J Pharmacol. 2021. PMID: 34126092 Free PMC article.
Cited by
-
Cangma Huadu granules attenuate H1N1 virus-induced severe lung injury correlated with repressed apoptosis and altered gut microbiome.Front Microbiol. 2022 Aug 8;13:947112. doi: 10.3389/fmicb.2022.947112. eCollection 2022. Front Microbiol. 2022. PMID: 36090063 Free PMC article.
-
Research and development of Chinese anti-COVID-19 drugs.Acta Pharm Sin B. 2022 Dec;12(12):4271-4286. doi: 10.1016/j.apsb.2022.09.002. Epub 2022 Sep 13. Acta Pharm Sin B. 2022. PMID: 36119967 Free PMC article. Review.
-
Efficacy and Safety of Re Du Ning Injection for Acute Exacerbations of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis.Evid Based Complement Alternat Med. 2022 Mar 21;2022:7479639. doi: 10.1155/2022/7479639. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35356238 Free PMC article. Review.
-
Efficacy and safety of aniseed powder for treating gastrointestinal symptoms of COVID-19: a randomized, placebo-controlled trial.Front Pharmacol. 2024 Jan 16;15:1331177. doi: 10.3389/fphar.2024.1331177. eCollection 2024. Front Pharmacol. 2024. PMID: 38292939 Free PMC article.
-
Efficacy and safety of add-on Viola odorata L. in the treatment of COVID-19: A randomized double-blind controlled trial.J Ethnopharmacol. 2023 Mar 25;304:116058. doi: 10.1016/j.jep.2022.116058. Epub 2022 Dec 17. J Ethnopharmacol. 2023. PMID: 36535329 Free PMC article. Clinical Trial.
References
-
- Assiri Abdullah, Al-Tawfiq Jaffar A., Al-Rabeeah Abdullah A., Al-Rabiah Fahad A., Al-Hajjar Sami, Al-Barrak Ali, Flemban Hesham, Al-Nassir Wafa N., Hanan H Balkhy, Al-Hakeem Rafat F., Hatem Q Makhdoom, Alimuddin I Zumla, Memish Ziad A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 2013;13:752–761. - PMC - PubMed
-
- Beigel J., Tomashek K., Dodd L., Mehta A., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C., ACTT-1 Study Group Members Remdesivir for the treatment of covid-19 -final report. N. Engl. J. Med. 2020;383:1813–1826. - PMC - PubMed
-
- Chang X.J., Zhang S., Chen J., Chen C.M., Zhou J., Wang Z.Z., Xiao W. Effect of reduning injection on acute lung injury of rats. Chin. J. Exp. Tradit. Med. Formulae. 2014;22:172–175.
-
- Chen J., Chang X.J., Chen C.M., Lu Y.Z., Zhou J., Wang Z.Z., Xiao W. Study on analgesic and antipyretics effect of re-du-ning injection. Modernization Tradit. Chin. Med. Materia Medica-World Sci. Technol. 2014;9:1912–1915.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

