B- and T-cell immune responses elicited by the Comirnaty® COVID-19 vaccine in nursing-home residents
- PMID: 34174397
- PMCID: PMC8223011
- DOI: 10.1016/j.cmi.2021.06.013
B- and T-cell immune responses elicited by the Comirnaty® COVID-19 vaccine in nursing-home residents
Abstract
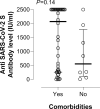
Objectives: The immunogenicity of the Comirnaty® vaccine against coronavirus disease 2019 (COVID-19) has not been adequately studied in elderly people with comorbidities. We assessed antibody and T-cell responses targeted to the S protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) following full vaccination in nursing-home residents.
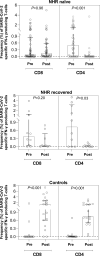
Methods: Sixty nursing-home residents (44 female, age 53-100 years), of whom ten had previously been diagnosed with COVID-19, and 18 healthy controls (15 female, age 27-54 years) were recruited. Pre- and post-vaccination blood specimens were available for quantification of total antibodies binding the SARS-CoV-2 S protein and for enumeration of SARS-CoV-2 S-reactive IFN-γ CD4+ and CD8+ T cells by flow cytometry.
Results: The seroconversion rate in (presumably) SARS-CoV-2-naïve nursing-home residents (41/43, 95.3%) was similar to that in controls (17/18, 94.4%). A booster effect was documented in post-vaccination samples of nursing-home residents with prior COVID-19. Plasma antibody levels were higher (p < 0.01) in recovered nursing-home residents (all 2500 IU/mL) than in individuals across the other two groups (median 1120 IU/mL in naïve nursing-home residents and 2211 IU/ml in controls). A large percentage of nursing-home residents had SARS-CoV-2 S-reactive IFN-γ CD8+ (naïve 31/49, 63.2%; recovered 8/10, 80%) or CD4+ T cells (naïve 35/49, 71.4%; recovered 7/10, 70%) at baseline, in contrast to healthy controls (3/17, 17.6% and 5/17, 29%, respectively). SARS-CoV-2 IFN-γ CD8+ and CD4+ T-cell responses were documented in 88% (15/17) and all control subjects after vaccination, respectively, but only in 65.5% (38/58) and 22.4% (13/58) of nursing-home residents. Overall, the median frequency of SARS-CoV-2 IFN-γ CD8+ and CD4+ T cells in nursing-home residents decreased in post-vaccination specimens, whereas it increased in controls.
Conclusion: The Comirnaty COVID-19 vaccine elicits robust SARS-CoV-2 S antibody responses in nursing-home residents. Nevertheless, the rate and frequency of detectable SARS-CoV-2 IFN-γ T-cell responses after vaccination was lower in nursing-home residents than in controls.
Keywords: Comirnaty®COVID-19 vaccine; Nursing-home residents; SARS-CoV-2; SARS-CoV-2 S T cells; SARS-CoV-2 S antibodies.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. - PubMed
-
- .
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

