In celebration of a century with insulin - Update of insulin gene mutations in diabetes
- PMID: 34174481
- PMCID: PMC8513141
- DOI: 10.1016/j.molmet.2021.101280
In celebration of a century with insulin - Update of insulin gene mutations in diabetes
Abstract
Background: While insulin has been central to the pathophysiology and treatment of patients with diabetes for the last 100 years, it has only been since 2007 that genetic variation in the INS gene has been recognised as a major cause of monogenic diabetes. Both dominant and recessive mutations in the INS gene are now recognised as important causes of neonatal diabetes and offer important insights into both the structure and function of insulin. It is also recognised that in rare cases, mutations in the INS gene can be found in patients with diabetes diagnosed outside the first year of life.
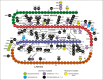
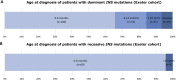
Scope of review: This review examines the genetics and clinical features of monogenic diabetes resulting from INS gene mutations from the first description in 2007 and includes information from 389 patients from 292 families diagnosed in Exeter with INS gene mutations. We discuss the implications for diagnosing and treating this subtype of monogenic diabetes.
Major conclusions: The dominant mutations in the INS gene typically affect the secondary structure of the insulin protein, usually by disrupting the 3 disulfide bonds in mature insulin. The resulting misfolded protein results in ER stress and beta-cell destruction. In contrast, recessive INS gene mutations typically result in no functional protein being produced due to reduced insulin biosynthesis or loss-of-function mutations in the insulin protein. There are clinical differences between the two genetic aetiologies, between the specific mutations, and within patients with identical mutations.
Keywords: Genetics; Insulin biosynthesis; Insulin gene; Monogenic diabetes; Neonatal diabetes; Pathophysiology.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures


References
-
- Bell G.I., Pictet R.L., Rutter W.J., Cordell B., Tischer E., Goodman H.M. Sequence of the human insulin gene. Nature. 1980;284(5751):26–32. - PubMed
-
- Bell G.I., Swain W.F., Pictet R., Cordell B., Goodman H.M., Rutter W.J. Nucleotide sequence of a cDNA clone encoding human preproinsulin. Nature. 1979;282(5738):525–527. - PubMed
-
- Hay C.W., Docherty K. Comparative analysis of insulin gene promoters: implications for diabetes research. Diabetes. 2006;55(12):3201–3213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

