Mechanics of ascending aortas from TGFβ-1, -2, -3 haploinsufficient mice and elastase-induced aortopathy
- PMID: 34174532
- PMCID: PMC8355174
- DOI: 10.1016/j.jbiomech.2021.110543
Mechanics of ascending aortas from TGFβ-1, -2, -3 haploinsufficient mice and elastase-induced aortopathy
Abstract
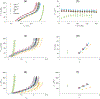
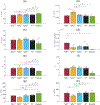
Transforming growth factor-beta (TGFβ-1, -2, -3) ligands act through a common receptor complex yet each is expressed in a unique and overlapping fashion throughout development. TGFβ plays a role in extra-cellular matrix composition with mutations to genes encoding TGFβ and TGFβ signaling molecules contributing to diverse and deadly thoracic aortopathies common in Loeys-Dietz syndrome (LDS). In this investigation, we studied the TGFβ ligand-specific mechanical phenotype of ascending thoracic aortas (ATA) taken from 4-to-6 months-old Tgfb1+/-, Tgfb2+/-, and Tgfb3+/- mice, their wild-type (WT) controls, and an elastase infusion model representative of severe elastolysis. Heterozygous mice were studied at an age without dilation to elucidate potential pre-aortopathic mechanical cues. Our findings indicate that ATAs from Tgfb2+/- mice demonstrated significant wall thickening, a corresponding decrease in biaxial stress, decreased biaxial stiffness, and a decrease in stored energy. These results were unlike the pathological elastase model where decreases in biaxial stretch were found along with increases in diameter, biaxial stress, and biaxial stiffness. ATAs from Tgfb1+/- and Tgfb3+/-, on the other hand, had few mechanical differences when compared to wild-type controls. Although aortopathy generally occurs later in development, our findings reveal that in 4-to-6 month-old animals, only Tgfb2+/- mice demonstrate a significant phenotype that fails to model ubiquitous elastolysis.
Keywords: Connective tissue disorders; Loeys-Dietz syndrome (LDS); Transforming growth factor-beta.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The authors declare no financial or nonfinancial conflicts of interest.
Figures
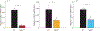
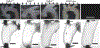


Similar articles
-
A missense TGFB2 variant p.(Arg320Cys) causes a paradoxical and striking increase in aortic TGFB1/2 expression.Eur J Hum Genet. 2016 Jan;25(1):157-160. doi: 10.1038/ejhg.2016.143. Epub 2016 Oct 26. Eur J Hum Genet. 2016. PMID: 27782106 Free PMC article.
-
Ectopia lentis in Loeys-Dietz syndrome type 4.Am J Med Genet A. 2020 Aug;182(8):1957-1959. doi: 10.1002/ajmg.a.61633. Epub 2020 May 28. Am J Med Genet A. 2020. PMID: 32462795
-
A mutation update on the LDS-associated genes TGFB2/3 and SMAD2/3.Hum Mutat. 2018 May;39(5):621-634. doi: 10.1002/humu.23407. Epub 2018 Mar 6. Hum Mutat. 2018. PMID: 29392890 Free PMC article.
-
Genotypic Categorization of Loeys-Dietz Syndrome Based on 24 Novel Families and Literature Data.Genes (Basel). 2019 Sep 28;10(10):764. doi: 10.3390/genes10100764. Genes (Basel). 2019. PMID: 31569402 Free PMC article. Review.
-
Transforming growth factor β signaling perturbation in the Loeys-Dietz syndrome.Curr Med Chem. 2012;19(3):454-60. doi: 10.2174/092986712803414286. Curr Med Chem. 2012. PMID: 22335518 Review.
Cited by
-
TGF-β2 enhances nanoscale cortex stiffness via condensation of cytoskeleton-focal adhesion plaque.Biophys J. 2025 Jan 21;124(2):336-350. doi: 10.1016/j.bpj.2024.12.007. Epub 2024 Dec 6. Biophys J. 2025. PMID: 39645584
-
Tracking an Elusive Killer: State of the Art of Molecular-Genetic Knowledge and Laboratory Role in Diagnosis and Risk Stratification of Thoracic Aortic Aneurysm and Dissection.Diagnostics (Basel). 2022 Jul 22;12(8):1785. doi: 10.3390/diagnostics12081785. Diagnostics (Basel). 2022. PMID: 35892496 Free PMC article. Review.
-
TGFβ-2 haploinsufficiency causes early death in mice with Marfan syndrome.Matrix Biol. 2023 Aug;121:41-55. doi: 10.1016/j.matbio.2023.05.004. Epub 2023 May 20. Matrix Biol. 2023. PMID: 37217119 Free PMC article.
-
Endothelial dysfunction promotes age-related reorganization of collagen fibers and alters aortic biomechanics in mice.Am J Physiol Heart Circ Physiol. 2025 Apr 1;328(4):H900-H914. doi: 10.1152/ajpheart.00056.2023. Epub 2025 Mar 10. Am J Physiol Heart Circ Physiol. 2025. PMID: 40062975 Free PMC article.
References
-
- Arthur HM, Bamforth SD, 2011. TGFbeta signaling and congenital heart disease: Insights from mouse studies. Birth Defects Res. A Clin. Mol. Teratol 91, 423–434. - PubMed
-
- Baek S, Gleason RL, Rajagopal KR, Humphrey JD, 2007. Theory of small on large: potential utility in computations of fluid–solid interactions in arteries. Comput. Methods Appl. Mech. Eng 196, 3070–3078.
-
- Bellini C, Bersi MR, Caulk AW, Ferruzzi J, Milewicz DM, Ramirez F, Rifkin DB, Tellides G, Yanagisawa H, Humphrey JD, 2017. Comparison of 10 murine models reveals a distinct biomechanical phenotype in thoracic aortic aneurysms. J. R. Soc. Interface 14, 20161036. 10.1098/rsif.2016.1036 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

