One swab, two tests: Validation of dual SARS-CoV-2 testing on the Abbott ID NOW™
- PMID: 34174710
- PMCID: PMC8196482
- DOI: 10.1016/j.jcv.2021.104896
One swab, two tests: Validation of dual SARS-CoV-2 testing on the Abbott ID NOW™
Abstract
Background: Point-of-care tests (POCT) are promising tools to detect SARS-CoV-2 in specific settings. Initial reports suggest the ID NOW™ COVID-19 assay (Abbott Diagnostics Inc, USA) is less sensitive than standard real-time reverse transcription polymerase chain reaction (rRT-PCR) assays. This has raised concern over false negatives in SARS-CoV-2 POCT.
Objectives: We compared the performance of the ID NOW™ COVID-19 assay to our in-house rRT-PCR assay to assess whether dry swabs used in ID NOW™ testing could be stored in transport media and be re-tested by rRT-PCR for redundancy and to provide material for further investigation.
Methods: Paired respiratory swabs collected from patients at three acute care hospitals were used. One swab in transport media (McMaster Molecular Media (MMM)) was tested for SARS-CoV-2 by a laboratory-developed two-target rRT-PCR assay. The second was stored dry in a sterile container and tested by the ID NOW™ COVID-19 assay. Following ID NOW™ testing, dry swabs were stored in MMM for up to 48 h and re-tested by rRT-PCR. Serially diluted SARS-CoV-2 particles were used to assess the impact of heat inactivation and storage time.
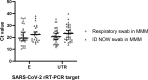
Results: Respiratory swabs (n = 343) from 179 individuals were included. Using rRT-PCR results as the comparator, the ID NOW™ COVID-19 assay had positive (PPA) and negative (NPA) percent agreements of 87.0% (95% CI:0.74-0.94) and 99.7% (95% CI:0.98-0.99). Re-tested swabs placed in MMM following ID NOW testing had PPA and NPA of 88.8% (95% CI:0.76-0.95) and 99.7% (95% CI:0.98-0.99), respectively.
Conclusions: Storing spent dry swabs in transport media for redundancy rRT-PCR testing is a potential approach to address possible false negatives with the ID NOW™ COVID-19 assay.
Keywords: Abbott ID NOW™ COVID-19; COVID-19; Point-of-care testing; SARS-CoV-2.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Characteristics of the ID-NOW™ test for the rapid detection of SARS-CoV-2.Epidemiol Mikrobiol Imunol. 2023 Winter;72(1):3-8. Epidemiol Mikrobiol Imunol. 2023. PMID: 37185021 English.
-
Abbott ID NOW™ COVID-19 assay: do not discard the swab.Diagn Microbiol Infect Dis. 2023 Apr;105(4):115832. doi: 10.1016/j.diagmicrobio.2022.115832. Epub 2022 Oct 13. Diagn Microbiol Infect Dis. 2023. PMID: 36731196 Free PMC article.
-
Clinical evaluation of a modified SARS-CoV-2 rapid molecular assay, ID NOW ™ COVID-19 2.0.J Infect Chemother. 2024 Sep;30(9):955-957. doi: 10.1016/j.jiac.2024.02.032. Epub 2024 Mar 2. J Infect Chemother. 2024. PMID: 38437982
-
Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS-CoV-2: A systematic review and meta-analysis.J Med Virol. 2021 Jul;93(7):4523-4531. doi: 10.1002/jmv.26994. Epub 2021 May 3. J Med Virol. 2021. PMID: 33913533 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
Cited by
-
Diagnostic accuracy of the Abbott ID NOW SARS-CoV-2 rapid test for the triage of acute medical admissions.J Hosp Infect. 2022 May;123:92-99. doi: 10.1016/j.jhin.2022.02.010. Epub 2022 Feb 23. J Hosp Infect. 2022. PMID: 35217130 Free PMC article.
-
Evaluating the Ability to ID (COVID-19) NOW: a Large Real-World Prospective Evaluation of the Abbott ID NOW COVID-19 Assay.Microbiol Spectr. 2022 Jun 29;10(3):e0051322. doi: 10.1128/spectrum.00513-22. Epub 2022 May 17. Microbiol Spectr. 2022. PMID: 35579469 Free PMC article.
References
-
- World Health Organization. 2021. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/. Last accessed February 16, 2021.
-
- Hengel B., Causer L., Matthews S., Smith K., Andrewartha K., Badman S., Spaeth B., Tangey A., Cunningham P., Phillips E., Ward J., Watts C., King J., Applegate T., Shephard M., Guy R. A decentralised point-of-care testing model to address inequities in the COVID-19 response. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30859-8. - DOI - PMC - PubMed
-
- Basu A., Zinger T., Inglima K., Woo K.M., Atie O., Yurasits L., See B., Aguero-Rosenfeld M.E. Performance of Abbott ID Now COVID-19 Rapid nucleic acid amplification test using nasopharyngeal swabs transported in viral transport media and dry nasal swabs in a New York City academic institution. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01136-20. e01136-20. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous