Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: An analysis of European data
- PMID: 34174723
- PMCID: PMC8220408
- DOI: 10.1016/j.jaut.2021.102685
Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: An analysis of European data
Abstract
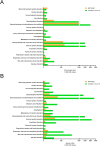
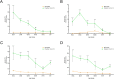
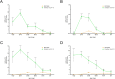
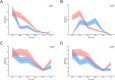
The involvement of viruses and SARS-CoV-2 in autoimmune diseases is well known. The recent demonstration that ChAdOx1 nCoV-19 Covid-19 (AstraZeneca) vaccine (ChA) favors the production of anti-platelet factor 4 (anti-PF4) antibodies, blood clots, and thrombocytopenia raises the question of whether other anti-CoViD-19 vaccines favor the same patterns of events. We assessed the frequency of severe adverse events (SAEs) documented in the EudraVigilance European database up to April 16, 2021 related to thrombocytopenia, bleeding, and blood clots in recipients of ChA compared to that of recipients of the BNT162b2 Covid-19 (Pfizer/BioNTech) vaccine (BNT). ChA administration was associated with a much higher frequency of SAEs in each AE Reaction Group as compared with that elicited by BNT. When considering AEs caused by thrombocytopenia, bleeding and blood clots, we observed 33 and 151 SAEs/1 million doses in BNT and ChA recipients, respectively. When considering patients with AEs related to cerebral/splanchnic venous thrombosis, and/or thrombocytopenia, we documented 4 and 30 SAEs and 0.4 and 4.8 deaths/1 million doses for BNT and ChA recipients, respectively. The highest risk following ChA vaccination is in young people and, likely, women of reproductive age, as suggested by hypothesized scenarios. In conclusion, the immune reaction promoted by ChA vaccine may lead to not only thrombocytopenia and cerebral/splanchnic venous thrombosis but also other thrombotic and thromboembolic SAEs. These events are not favored by BNT vaccine. Our study may help in the evaluation of the benefit/risk profile of the ChA vaccine considering the epidemic curve present in a country.
Keywords: Anti-CoViD-19 vaccines; Risk factors; Severe adverse events; Thrombocytopenia; Venous thrombosis.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
The authors have no relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. All this includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, and royalties.
Figures
References
-
- Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., Manry J., Shaw E., Haljasmägi L., Peterson P., Lorenzo L., Bizien L., Trouillet-Assant S., Dobbs K., de Jesus A.A., Belot A., Kallaste A., Catherinot E., Tandjaoui-Lambiotte Y., Le Pen J., Kerner G., Bigio B., Seeleuthner Y., Yang R., Bolze A., Spaan A.N., Delmonte O.M., Abers M.S., Aiuti A., Casari G., Lampasona V., Piemonti L., Ciceri F., Bilguvar K., Lifton R.P., Vasse M., Smadja D.M., Migaud M., Hadjadj J., Terrier B., Duffy D., Quintana-Murci L., van de Beek D., Roussel L., Vinh D.C., Tangye S.G., Haerynck F., Dalmau D., Martinez-Picado J., Brodin P., Nussenzweig M.C., Boisson-Dupuis S., Rodríguez-Gallego C., Vogt G., Mogensen T.H., Oler A.J., Gu J., Burbelo P.D., Cohen J.I., Biondi A., Bettini L.R., D'Angio M., Bonfanti P., Rossignol P., Mayaux J., Rieux-Laucat F., Husebye E.S., Fusco F., Ursini M.V., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Castagnoli R., Montagna D., Licari A., Marseglia G.L., Duval X., Ghosn J., Tsang J.S., Goldbach-Mansky R., Kisand K., Lionakis M.S., Puel A., Zhang S.-Y., Holland S.M., Gorochov G., Jouanguy E., Rice C.M., Cobat A., Notarangelo L.D., Abel L., Su H.C., Casanova J.-L. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;80–:370. doi: 10.1126/science.abd4585. eabd4585. - DOI - PMC - PubMed
-
- Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., Sule G., Gockman K., Madison J.A., Zuo M., Yadav V., Wang J., Woodard W., Lezak S.P., Lugogo N.L., Smith S.A., Morrissey J.H., Kanthi Y., Knight J.S. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abd3876. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

