Trends in endocrine therapy prescription and survival in patients with non-metastatic hormone receptor positive breast cancer treated with endocrine therapy: A population based-study
- PMID: 34174766
- PMCID: PMC8242053
- DOI: 10.1016/j.breast.2021.06.003
Trends in endocrine therapy prescription and survival in patients with non-metastatic hormone receptor positive breast cancer treated with endocrine therapy: A population based-study
Abstract
Purpose: To identify prognostic factors of invasive-disease free survival (iDFS) in women with non-metastatic hormone receptor positive (HR+) breast cancer (BC) in daily routine practice.
Methods: We performed a retrospective study using data from the Côte d'Or breast and gynecological cancer registry in France. All women diagnosed with primary invasive non-metastatic HR + BC from 1998 to 2015 and treated by endocrine therapy (ET) were included. Women with bilateral tumors or who received ET for either metastasis or relapse were excluded. We performed adjusted survival analysis and Cox regression to identify prognostic factors of iDFS.
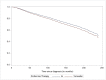
Results: A total of 3976 women were included. Age at diagnosis, ET class, SBR grade, treatment, stage and comorbidity were independently associated with iDFS. Women who had neither surgery nor radiotherapy had the highest risk of recurrence (HR = 3.75, 95%CI [2.65-5.32], p < 0.0001). Receiving aromatase inhibitors (AI) was associated with a lower risk of recurrence (HR = 0.70, 95%CI [0.54-0.90], p = 0.055) compared to tamoxifen. Compared to women with no comorbidities, women with 1 or 2 comorbidities were more likely to receive AI (OR = 1.63, 95%CI [1.22-2.17], p = 0.0009).
Conclusions: Comorbidities, age at diagnosis and previous treatment were associated with iDFS in non-metastatic HR + BC patients. This study also showed that women who received tamoxifen for their cancer experienced worse iDFS compared to women treated with AI.
Keywords: Breast cancer; Endocrine therapy; Hormone receptor positive; Invasive disease-free survival; Non-metastatic cancer.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures
Similar articles
-
Luteininzing hormone releasing hormones analogs in combination with tamoxifen for the adjuvant treatment of premenopausal women with hormone receptor positive breast cancer.Expert Opin Pharmacother. 2017 Sep;18(13):1357-1362. doi: 10.1080/14656566.2017.1363181. Epub 2017 Aug 10. Expert Opin Pharmacother. 2017. PMID: 28764603 Review.
-
OFS plus AI or SERM vs. SERM alone in premenopausal women with hormone receptor-positive breast cancer: a prospective cohort study using the real-world database.Breast Cancer. 2019 May;26(3):339-348. doi: 10.1007/s12282-018-0929-6. Epub 2018 Oct 26. Breast Cancer. 2019. PMID: 30367358
-
Local estrogen therapy and risk of breast cancer recurrence among hormone-treated patients: a nested case-control study.Breast Cancer Res Treat. 2012 Sep;135(2):603-9. doi: 10.1007/s10549-012-2198-y. Epub 2012 Aug 19. Breast Cancer Res Treat. 2012. PMID: 22903687
-
Survival Outcomes of Early-Stage Hormone Receptor-Positive Breast Cancer in Elderly Women.Ann Surg Oncol. 2020 Nov;27(12):4853-4860. doi: 10.1245/s10434-020-08945-1. Epub 2020 Sep 11. Ann Surg Oncol. 2020. PMID: 32918178
-
Sequential hormonal therapy for metastatic breast cancer after adjuvant tamoxifen or anastrozole.Breast Cancer Res Treat. 2003;80 Suppl 1:S19-26; discussion S27-8. doi: 10.1023/a:1025459232293. Breast Cancer Res Treat. 2003. PMID: 14535531 Review.
References
-
- Howell A. The “Arimidex”, Tamoxifen, Alone or in Combination (ATAC) Trial: a step forward in the treatment of early breast cancer. Rev Recent Clin Trials. 2006;1(3):207–215. Sep. - PubMed
-
- A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747–2757. Dec 29. - PubMed
-
- Derks M.G.M., Blok E.J., Seynaeve C., Nortier J.W.R., Kranenbarg E.M.-K., Liefers G.-J. Adjuvant tamoxifen and exemestane in women with postmenopausal early breast cancer (TEAM): 10-year follow-up of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(9):1211–1220. Sep 1. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical