Clinicopathologic features of TDO2 overexpression in renal cell carcinoma
- PMID: 34174844
- PMCID: PMC8236178
- DOI: 10.1186/s12885-021-08477-1
Clinicopathologic features of TDO2 overexpression in renal cell carcinoma
Abstract
Background: Tryptophan 2,3-dioxygenase (TDO2) is the primary enzyme catabolizing tryptophan. Several lines of evidence revealed that overexpression of TDO2 is involved in anoikis resistance, spheroid formation, proliferation, and invasion and correlates with poor prognosis in some cancers. The aim of this research was to uncover the expression and biofunction of TDO2 in renal cell carcinoma (RCC).
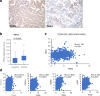
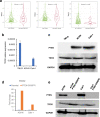
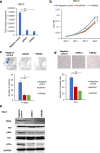
Methods: To show the expression of TDO2 in RCC, we performed qRT-PCR and immunohistochemistry in integration with TCGA data analysis. The interaction of TDO2 with PD-L1, CD44, PTEN, and TDO2 expression was evaluated. We explored proliferation, colony formation, and invasion in RCC cells line affected by knockdown of TDO2.
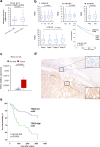
Results: RNA-Seq and immunohistochemical analysis showed that TDO2 expression was upregulated in RCC tissues and was associated with advanced disease and poor survival of RCC patients. Furthermore, TDO2 was co-expressed with PD-L1 and CD44. In silico analysis and in vitro knockout of PTEN in RCC cell lines revealed the ability of PTEN to regulate the expression of TDO2. Knockdown of TDO2 suppressed the proliferation and invasion of RCC cells.
Conclusion: Our results suggest that TDO2 might have an important role in disease progression and could be a promising marker for targeted therapy in RCC. (199 words).
Keywords: Cancer stem cell; PD-L1; PTEN; Renal cell carcinoma; TDO2.
Conflict of interest statement
The authors report no potential conflicts of interest.
Figures
Similar articles
-
TDO2 Overexpression Is Associated with Cancer Stem Cells and Poor Prognosis in Esophageal Squamous Cell Carcinoma.Oncology. 2018;95(5):297-308. doi: 10.1159/000490725. Epub 2018 Aug 22. Oncology. 2018. PMID: 30134247
-
TDO2 overexpression correlates with poor prognosis, cancer stemness, and resistance to cetuximab in bladder cancer.Cancer Rep (Hoboken). 2021 Dec;4(6):e1417. doi: 10.1002/cnr2.1417. Epub 2021 Jun 7. Cancer Rep (Hoboken). 2021. PMID: 34101386 Free PMC article.
-
Essential Roles of TDO2 in Gastric Cancer: TDO2 Is Associated with Cancer Progression, Patient Survival, PD-L1 Expression, and Cancer Stem Cells.Pathobiology. 2023;90(1):44-55. doi: 10.1159/000523750. Epub 2022 Jun 9. Pathobiology. 2023. PMID: 35679834
-
BUB1B Overexpression Is an Independent Prognostic Marker and Associated with CD44, p53, and PD-L1 in Renal Cell Carcinoma.Oncology. 2021;99(4):240-250. doi: 10.1159/000512446. Epub 2021 Feb 15. Oncology. 2021. PMID: 33588420
-
Circular RNA circZNF566 promotes hepatocellular carcinoma progression by sponging miR-4738-3p and regulating TDO2 expression.Cell Death Dis. 2020 Jun 12;11(6):452. doi: 10.1038/s41419-020-2616-8. Cell Death Dis. 2020. PMID: 32532962 Free PMC article.
Cited by
-
Pancancer Analysis of Revealed TDO2 as a Biomarker of Prognosis and Immunotherapy.Dis Markers. 2022 Sep 9;2022:5447017. doi: 10.1155/2022/5447017. eCollection 2022. Dis Markers. 2022. PMID: 36118672 Free PMC article.
-
Dexamethasone Promotes a Stem-Like Phenotype in Human Melanoma Cells via Tryptophan 2,3 Dioxygenase.Front Pharmacol. 2022 Jun 30;13:911019. doi: 10.3389/fphar.2022.911019. eCollection 2022. Front Pharmacol. 2022. PMID: 35847038 Free PMC article.
-
Mechanics of serotonin-producing human entero-endocrine cells.Mechanobiol Med. 2024 Feb 8;2(2):100044. doi: 10.1016/j.mbm.2024.100044. eCollection 2024 Jun. Mechanobiol Med. 2024. PMID: 40395857 Free PMC article.
-
TDO2 + cancer-associated fibroblasts mediate cutaneous squamous cell carcinoma immune escape via impeding infiltration of CD8 + T cells.Cancer Immunol Immunother. 2025 Jan 3;74(2):67. doi: 10.1007/s00262-024-03921-0. Cancer Immunol Immunother. 2025. PMID: 39751882 Free PMC article.
-
Whole transcriptome expression profiles in kidney samples from rats with hyperuricaemic nephropathy.PLoS One. 2022 Dec 19;17(12):e0276591. doi: 10.1371/journal.pone.0276591. eCollection 2022. PLoS One. 2022. PMID: 36534664 Free PMC article.
References
-
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F (2020) Global Cancer Observatory: Cancer Today [https://gco.iarc.fr/today]. Accessed 04 Jan 2021.
-
- Choueiri TK, Motzer RJ. Systemic Therapy for Metastatic Renal-Cell Carcinoma. The N Engl J Med. 2017;376(4):354-66. 10.1007/s11306-017-1288-6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

