Beyond the lesion site: minocycline augments inflammation and anxiety-like behavior following SCI in rats through action on the gut microbiota
- PMID: 34174901
- PMCID: PMC8234629
- DOI: 10.1186/s12974-021-02123-0
Beyond the lesion site: minocycline augments inflammation and anxiety-like behavior following SCI in rats through action on the gut microbiota
Abstract
Background: Minocycline is a clinically available synthetic tetracycline derivative with anti-inflammatory and antibiotic properties. The majority of studies show that minocycline can reduce tissue damage and improve functional recovery following central nervous system injuries, mainly attributed to the drug's direct anti-inflammatory, anti-oxidative, and neuroprotective properties. Surprisingly the consequences of minocycline's antibiotic (i.e., antibacterial) effects on the gut microbiota and systemic immune response after spinal cord injury have largely been ignored despite their links to changes in mental health and immune suppression.
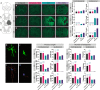
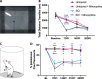
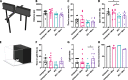
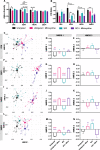
Methods: Here, we sought to determine minocycline's effect on spinal cord injury-induced changes in the microbiota-immune axis using a cervical contusion injury in female Lewis rats. We investigated a group that received minocycline following spinal cord injury (immediately after injury for 7 days), an untreated spinal cord injury group, an untreated uninjured group, and an uninjured group that received minocycline. Plasma levels of cytokines/chemokines and fecal microbiota composition (using 16s rRNA sequencing) were monitored for 4 weeks following spinal cord injury as measures of the microbiota-immune axis. Additionally, motor recovery and anxiety-like behavior were assessed throughout the study, and microglial activation was analyzed immediately rostral to, caudal to, and at the lesion epicenter.
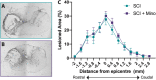
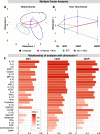
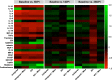
Results: We found that minocycline had a profound acute effect on the microbiota diversity and composition, which was paralleled by the subsequent normalization of spinal cord injury-induced suppression of cytokines/chemokines. Importantly, gut dysbiosis following spinal cord injury has been linked to the development of anxiety-like behavior, which was also decreased by minocycline. Furthermore, although minocycline attenuated spinal cord injury-induced microglial activation, it did not affect the lesion size or promote measurable motor recovery.
Conclusion: We show that minocycline's microbiota effects precede its long-term effects on systemic cytokines and chemokines following spinal cord injury. These results provide an exciting new target of minocycline as a therapeutic for central nervous system diseases and injuries.
Keywords: Inflammation; Microbiota; Minocycline; Spinal cord injury.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Weaver LC, Marsh DR, Gris D, Brown A, Dekaban GA. Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention. Prog Brain Res. 2006:245–63 Elsevier. [cited 2020 Oct 20]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0079612305520168. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

