A conserved female-specific larval requirement for MtnB function facilitates sex separation in multiple species of disease vector mosquitoes
- PMID: 34174948
- PMCID: PMC8234664
- DOI: 10.1186/s13071-021-04844-w
A conserved female-specific larval requirement for MtnB function facilitates sex separation in multiple species of disease vector mosquitoes
Abstract
Background: Clusters of sex-specific loci are predicted to shape the boundaries of the M/m sex-determination locus of the dengue vector mosquito Aedes aegypti, but the identities of these genes are not known. Identification and characterization of these loci could promote a better understanding of mosquito sex chromosome evolution and lead to the elucidation of new strategies for male mosquito sex separation, a requirement for several emerging mosquito population control strategies that are dependent on the mass rearing and release of male mosquitoes. This investigation revealed that the methylthioribulose-1-phosphate dehydratase (MtnB) gene, which resides adjacent to the M/m locus and encodes an evolutionarily conserved component of the methionine salvage pathway, is required for survival of female larvae.
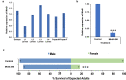
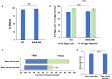
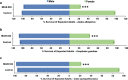
Results: Larval consumption of Saccharomyces cerevisiae (yeast) strains engineered to express interfering RNA corresponding to MtnB resulted in target gene silencing and significant female death, yet had no impact on A. aegypti male survival or fitness. Integration of the yeast larvicides into mass culturing protocols permitted scaled production of fit adult male mosquitoes. Moreover, silencing MtnB orthologs in Aedes albopictus, Anopheles gambiae, and Culex quinquefasciatus revealed a conserved female-specific larval requirement for MtnB among different species of mosquitoes.
Conclusions: The results of this investigation, which may have important implications for the study of mosquito sex chromosome evolution, indicate that silencing MtnB can facilitate sex separation in multiple species of disease vector insects.
Keywords: Aedes aegypti; Aedes albopictus; Anopheles gambiae; Culex quinquefasciatus; Larvae; Larvicide; Methionine; RNAi; Saccharomyces cerevisiae; Sex; Yeast.
Conflict of interest statement
MDS was named as the inventor on U.S. patent application number 62/751,052. The pending application did not impact her data interpretation or decision to publish this work. All other authors declare that they lack competing interests.
Figures
Similar articles
-
A Conserved Female-Specific Requirement for the GGT Gene in Mosquito Larvae Facilitates RNAi-Mediated Sex Separation in Multiple Species of Disease Vector Mosquitoes.Pathogens. 2022 Jan 27;11(2):169. doi: 10.3390/pathogens11020169. Pathogens. 2022. PMID: 35215113 Free PMC article.
-
Characterization of a yeast interfering RNA larvicide with a target site conserved in the synaptotagmin gene of multiple disease vector mosquitoes.PLoS Negl Trop Dis. 2019 May 20;13(5):e0007422. doi: 10.1371/journal.pntd.0007422. eCollection 2019 May. PLoS Negl Trop Dis. 2019. PMID: 31107878 Free PMC article.
-
Characterization of a broad-based mosquito yeast interfering RNA larvicide with a conserved target site in mosquito semaphorin-1a genes.Parasit Vectors. 2019 May 22;12(1):256. doi: 10.1186/s13071-019-3504-x. Parasit Vectors. 2019. PMID: 31118082 Free PMC article.
-
A functional requirement for sex-determination M/m locus region lncRNA genes in Aedes aegypti female larvae.Sci Rep. 2021 May 20;11(1):10657. doi: 10.1038/s41598-021-90194-7. Sci Rep. 2021. PMID: 34017069 Free PMC article.
-
RNA Interference for Mosquito and Mosquito-Borne Disease Control.Insects. 2017 Jan 5;8(1):4. doi: 10.3390/insects8010004. Insects. 2017. PMID: 28067782 Free PMC article. Review.
Cited by
-
VectorBase.org updates: bioinformatic resources for invertebrate vectors of human pathogens and related organisms.Curr Opin Insect Sci. 2022 Apr;50:100860. doi: 10.1016/j.cois.2021.11.008. Epub 2021 Dec 3. Curr Opin Insect Sci. 2022. PMID: 34864248 Free PMC article. Review.
-
A Broad-Based Mosquito Yeast Interfering RNA Pesticide Targeting Rbfox1 Represses Notch Signaling and Kills Both Larvae and Adult Mosquitoes.Pathogens. 2021 Sep 28;10(10):1251. doi: 10.3390/pathogens10101251. Pathogens. 2021. PMID: 34684200 Free PMC article.
-
A Conserved Female-Specific Requirement for the GGT Gene in Mosquito Larvae Facilitates RNAi-Mediated Sex Separation in Multiple Species of Disease Vector Mosquitoes.Pathogens. 2022 Jan 27;11(2):169. doi: 10.3390/pathogens11020169. Pathogens. 2022. PMID: 35215113 Free PMC article.
-
Selective targeting of biting females to control mosquito-borne infectious diseases.Trends Parasitol. 2022 Sep;38(9):791-804. doi: 10.1016/j.pt.2022.05.012. Epub 2022 Jun 13. Trends Parasitol. 2022. PMID: 35952630 Free PMC article. Review.
-
Requirements for market entry of gene drive-modified mosquitoes for control of vector-borne diseases: analogies to other biologic and biotechnology products.Front Bioeng Biotechnol. 2023 Jun 8;11:1205865. doi: 10.3389/fbioe.2023.1205865. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37362219 Free PMC article. Review.
References
-
- Centers for Disease Control CDC. Mosquitoes and diseases: A-Z. https://www.cdc.gov/mosquitoes/about/diseases.html. Accessed Jan 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

