Application of weighting methods for presenting risk-of-bias assessments in systematic reviews of diagnostic test accuracy studies
- PMID: 34174958
- PMCID: PMC8236145
- DOI: 10.1186/s13643-021-01744-z
Application of weighting methods for presenting risk-of-bias assessments in systematic reviews of diagnostic test accuracy studies
Abstract
Background: An assessment of the validity of individual diagnostic accuracy studies in systematic reviews is necessary to guide the analysis and the interpretation of results. Such an assessment is performed for each included study and typically reported at the study level. As studies may differ in sample size and disease prevalence, with larger studies contributing more to the meta-analysis, such a study-level report does not always reflect the risk of bias in the total body of evidence. We aimed to develop improved methods of presenting the risk of bias in the available evidence on diagnostic accuracy of medical tests in systematic reviews, reflecting the relative contribution of the study to the body of evidence in the review.
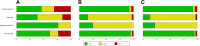
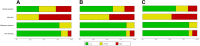
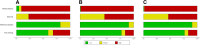
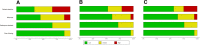
Methods: We applied alternative methods to represent evaluations with the Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2), weighting studies according to their relative contribution to the total sample size or their relative effective sample size. We used these methods in four existing systematic reviews of diagnostic accuracy studies, including 9, 13, 22, and 32 studies, respectively.
Results: The risk-of-bias summaries for each domain of the QUADAS-2 checklist changed in all four sets of studies after replacing unit weights for the studies with relative sample sizes or with the relative effective sample size. As an example, the risk of bias was high in the patient selection domain in 31% of the studies in one review, unclear in 23% and low in 46% of studies. Weighting studies according to the relative sample size changed the corresponding proportions to 4%, 4%, and 92%, respectively. The difference between the two weighting methods was small and more noticeable when the reviews included a smaller number of studies with wider range of sample size.
Conclusions: We present an alternative way of presenting the results of risk-of-bias assessments in systematic reviews of diagnostic accuracy studies. Weighting studies according to their relative sample size or their relative effective sample size can be used as more informative summaries of the risk of bias in the total body of available evidence.
Systematic review registrations: Not applicable.
Keywords: Diagnostic accuracy studies; Quality appraisal; Risk-of-bias assessment; Systematic reviews.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
High and unclear risk of bias assessments are predominant in diagnostic accuracy studies included in Cochrane reviews.J Clin Epidemiol. 2018 Sep;101:73-78. doi: 10.1016/j.jclinepi.2018.05.001. Epub 2018 May 16. J Clin Epidemiol. 2018. PMID: 29777798
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.Health Technol Assess. 2005 Mar;9(12):1-113, iii. doi: 10.3310/hta9120. Health Technol Assess. 2005. PMID: 15774235 Review.
-
[Risk on bias assessment: (6) A Revised Tool for the Quality Assessment on Diagnostic Accuracy Studies (QUADAS-2)].Zhonghua Liu Xing Bing Xue Za Zhi. 2018 Apr 10;39(4):524-531. doi: 10.3760/cma.j.issn.0254-6450.2018.04.028. Zhonghua Liu Xing Bing Xue Za Zhi. 2018. PMID: 29699051 Chinese.
-
Use of B-Type Natriuretic Peptide (BNP) and N-Terminal proBNP (NT-proBNP) as Diagnostic Tests in Adults With Suspected Heart Failure: A Health Technology Assessment.Ont Health Technol Assess Ser. 2021 May 6;21(2):1-125. eCollection 2021. Ont Health Technol Assess Ser. 2021. PMID: 34055110 Free PMC article.
Cited by
-
Clinical Outcomes of 3D-Printed Titanium Patient-Specific Implants in Lumbar Interbody Fusion: A Prospective Clinical Trial with a Systematic Review of Conventional Techniques.J Pers Med. 2025 Jul 16;15(7):320. doi: 10.3390/jpm15070320. J Pers Med. 2025. PMID: 40710437 Free PMC article.
-
A Systematic Review of Recreational Nitrous Oxide Use: Implications for Policy, Service Delivery and Individuals.Int J Environ Res Public Health. 2022 Sep 14;19(18):11567. doi: 10.3390/ijerph191811567. Int J Environ Res Public Health. 2022. PMID: 36141850 Free PMC article.
-
Effectiveness of workplace health promotion programs for industrial workers: a systematic review.BMC Public Health. 2025 Jan 15;25(1):168. doi: 10.1186/s12889-025-21365-8. BMC Public Health. 2025. PMID: 39815242 Free PMC article.
-
A meta-analysis investigating the outcomes and correlation between heart rate variability biofeedback training on depressive symptoms and heart rate variability outcomes versus standard treatment in comorbid adult populations.Acta Biomed. 2023 Aug 3;94(4):e2023214. doi: 10.23750/abm.v94i4.14305. Acta Biomed. 2023. PMID: 37539604 Free PMC article. Review.
-
Chronic kidney disease in adults with sickle cell trait: a systematic review and meta-analysis.Blood Adv. 2025 Jul 22;9(14):3644-3657. doi: 10.1182/bloodadvances.2025015920. Blood Adv. 2025. PMID: 40300155 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

