Antibody and B cell responses to SARS-CoV-2 infection and vaccination
- PMID: 34174992
- PMCID: PMC8233571
- DOI: 10.1016/j.chom.2021.06.009
Antibody and B cell responses to SARS-CoV-2 infection and vaccination
Abstract
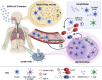
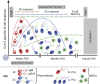
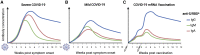
Antibodies, and the B cell and plasma cell populations responsible for their production, are key components of the human immune system's response to SARS-CoV-2, which has caused the coronavirus disease 2019 (COVID-19) pandemic. Here, we review findings addressing the nature of antibody responses against SARS-CoV-2 and their role in protecting from infection or modulating COVID-19 disease severity. In just over a year, much has been learned, and replicated in independent studies, about human immune responses to this pathogen, contributing to the development of effective vaccines. Nevertheless, important questions remain about the duration and effectiveness of antibody responses, differences between immunity derived from infection compared to vaccination, the cellular basis for serological findings, and the extent to which viral variants will escape from current immunity.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests S.D.B. has consulted for Regeneron, Sanofi, and Novartis on topics unrelated to this manuscript, and owns stock in AbCellera Biologics.
Figures

References
-
- Aldridge R.W., Lewer D., Beale S., Johnson A.M., Zambon M., Hayward A.C., Fragaszy E.B., Flu Watch Group Seasonality and immunity to laboratory-confirmed seasonal coronaviruses (HCoV-NL63, HCoV-OC43, and HCoV-229E): results from the Flu Watch cohort study. Wellcome Open Res. 2020;5:52. - PMC - PubMed
-
- Anderson E.M., Goodwin E.C., Verma A., Arevalo C.P., Bolton M.J., Weirick M.E., Gouma S., McAllister C.M., Christensen S.R., Weaver J., et al. UPenn COVID Processing Unit Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. 2021;184:1858–1864.e10. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

