Oxidative decomposition and mineralization of caffeine by advanced oxidation processes: The effect of hybridization
- PMID: 34175811
- PMCID: PMC8237590
- DOI: 10.1016/j.ultsonch.2021.105635
Oxidative decomposition and mineralization of caffeine by advanced oxidation processes: The effect of hybridization
Abstract
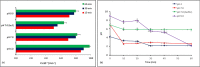
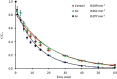
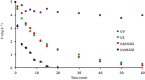
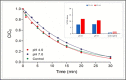
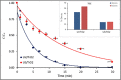
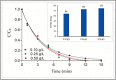
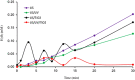
The study consists of a detailed investigation of the degradability of the emerging water contaminant-caffeine by homogeneous and heterogeneous Advanced Oxidation Processes (AOP's), estimation of a synergy index for each hybrid operation thereof, and proposing the most plausible reaction mechanisms that are consistent with the experimental data. It also encompasses evaluation of the effect of the water matrix represented by carbonate species and humic acids, as strong scavengers of hydroxyl radicals. The results showed that single AOP's such as sonolysis (577 kHz) and photolysis with H2O2 provided complete caffeine elimination, but they were insufficient for the mineralization of the compound. Hybrid AOP's were considerably more effective, particularly when operated at a heterogeneous mode using commercial TiO2. The most effective hybrid process was UV-H2O2/TiO2, which provided more than 75% TOC decay at the minimum test doses of the reagent and catalyst. While the addition of ultrasound to the process significantly increased the rate of caffeine decomposition, it reduced the overall degradation of the compound to 64% in terms of TOC decay. The antagonistic effect was attributed to the formation of excess H2O2, and the presence of cavity clouds and/or high density layers that inhibited the transmission of UV light. The effect of natural water ingredients was found to reduce the reaction rates, signifying the major contribution of hydroxyl radicals to the destruction of caffeine. The proposed reaction mechanisms based on OH radical attack and the calculated energy barriers were in good agreement with the experimentally detected reaction byproducts.
Keywords: AOP’s; Cavitation; Energy barrier; Hybrid; OH; Synergy index.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Załęska-Radziwiłł M., Affek K., Rybak J. Ecotoxicity of chosen pharmaceuticals in relation to micro-organisms-risk assessment. Desalin. Water Treat. 2014;52(19-21):3908–3917. doi: 10.1080/19443994.2014.887503. - DOI
-
- Bruton T., Alboloushi A., De La Garza B., Kim B.O., Halden R.U. Fate of caffeine in the environment and ecotoxicological considerations. ACS Symp. Ser. 2010
-
- Gardinali P.R., Zhao X.u. Trace determination of caffeine in surface water samples by liquid chromatography–atmospheric pressure chemical ionization–mass spectrometry (LC-APCI-MS) Environ. Int. 2002;28(6):521–528. - PubMed
-
- Sotelo J.L., Ovejero G., Rodríguez A., Álvarez S., Galán J., García J. Competitive adsorption studies of caffeine and diclofenac aqueous solutions by activated carbon. Chem. Eng. J. 2014;240:443–453.
-
- J.L. Sotelo, G. Ovejero, A. Rodríguez, S. Álvarez, J. García, Study of Natural Clay Adsorbent Sepiolite for the Removal of Caffeine from Aqueous Solutions : Batch and Fixed-Bed Column Operation, (2013). DOI:10.1007/s11270-013-1466-8.
LinkOut - more resources
Full Text Sources

