Erythropoietin monotherapy for neuroprotection after neonatal encephalopathy in low-to-middle income countries: a systematic review and meta-analysis
- PMID: 34175900
- PMCID: PMC8440196
- DOI: 10.1038/s41372-021-01132-4
Erythropoietin monotherapy for neuroprotection after neonatal encephalopathy in low-to-middle income countries: a systematic review and meta-analysis
Abstract
Objective: We examined whether erythropoietin monotherapy improves neurodevelopmental outcomes in near-term and term infants with neonatal encephalopathy (NE) in low-middle income countries (LMICs).
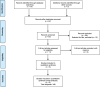
Methods: We searched Pubmed, Embase, and Web of Science databases to identify studies that used erythropoietin (1500-12,500 units/kg/dose) or a derivative to treat NE.
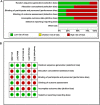
Results: Five studies, with a total of 348 infants in LMICs, were retrieved. However, only three of the five studies met the primary outcome of death or neuro-disability at 18 months of age or later. Erythropoietin reduced the risk of death (during the neonatal period and at follow-up) or neuro-disability at 18 months or later (p < 0.05). Death or neuro-disability occurred in 27.6% of the erythropoietin group and 49.7% of the comparison group (risk ratio 0.56 (95% CI: 0.42-0.75)).
Conclusion: The pooled data suggest that erythropoietin monotherapy may improve outcomes after NE in LMICs where therapeutic hypothermia is not available.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

