COVID-19 Infection and Circulating Microparticles-Reviewing Evidence as Microthrombogenic Risk Factor for Cerebral Small Vessel Disease
- PMID: 34176095
- PMCID: PMC8235918
- DOI: 10.1007/s12035-021-02457-z
COVID-19 Infection and Circulating Microparticles-Reviewing Evidence as Microthrombogenic Risk Factor for Cerebral Small Vessel Disease
Abstract
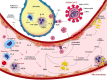
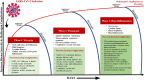
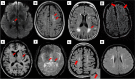
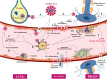
Severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) due to novel coronavirus disease 2019 (COVID-19) has affected the global society in numerous unprecedented ways, with considerable morbidity and mortality. Both direct and indirect consequences from COVID-19 infection are recognized to give rise to cardio- and cerebrovascular complications. Despite current limited knowledge on COVID-19 pathogenesis, inflammation, endothelial dysfunction, and coagulopathy appear to play critical roles in COVID-19-associated cerebrovascular disease (CVD). One of the major subtypes of CVD is cerebral small vessel disease (CSVD) which represents a spectrum of pathological processes of various etiologies affecting the brain microcirculation that can trigger subsequent neuroinflammation and neurodegeneration. Prevalent with aging, CSVD is a recognized risk factor for stroke, vascular dementia, and Alzheimer's disease. In the background of COVID-19 infection, the heightened cellular activations from inflammations and oxidative stress may result in elevated levels of microthrombogenic extracellular-derived circulating microparticles (MPs). Consequently, MPs could act as pro-coagulant risk factor that may serve as microthrombi for the vulnerable microcirculation in the brain leading to CSVD manifestations. This review aims to appraise the accumulating body of evidence on the plausible impact of COVID-19 infection on the formation of microthrombogenic MPs that could lead to microthrombosis in CSVD manifestations, including occult CSVD which may last well beyond the pandemic era.
Keywords: COVID-19; Cerebral small vessel disease; Coagulopathy; Microparticles; Stroke.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Review of the mechanism of infection induced cerebral small vessel disease.Front Immunol. 2025 May 26;16:1594891. doi: 10.3389/fimmu.2025.1594891. eCollection 2025. Front Immunol. 2025. PMID: 40491910 Free PMC article. Review.
-
Diets and Cellular-Derived Microparticles: Weighing a Plausible Link With Cerebral Small Vessel Disease.Front Cardiovasc Med. 2021 Feb 24;8:632131. doi: 10.3389/fcvm.2021.632131. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33718454 Free PMC article. Review.
-
Increased circulating microparticles contribute to severe infection and adverse outcomes of COVID-19 in patients with diabetes.Am J Physiol Heart Circ Physiol. 2022 Dec 1;323(6):H1176-H1193. doi: 10.1152/ajpheart.00409.2022. Epub 2022 Oct 21. Am J Physiol Heart Circ Physiol. 2022. PMID: 36269646 Free PMC article.
-
Hypertension-Induced Cerebral Small Vessel Disease Leading to Cognitive Impairment.Chin Med J (Engl). 2018 Mar 5;131(5):615-619. doi: 10.4103/0366-6999.226069. Chin Med J (Engl). 2018. PMID: 29483399 Free PMC article. Review.
-
Emerging Role of Immunity in Cerebral Small Vessel Disease.Front Immunol. 2018 Jan 25;9:67. doi: 10.3389/fimmu.2018.00067. eCollection 2018. Front Immunol. 2018. PMID: 29422904 Free PMC article. Review.
Cited by
-
Procoagulant Microvesicles in COVID-19 Patients: Possible Modulators of Inflammation and Prothrombotic Tendency.Infect Drug Resist. 2022 Apr 29;15:2359-2368. doi: 10.2147/IDR.S355395. eCollection 2022. Infect Drug Resist. 2022. PMID: 35517897 Free PMC article.
-
Circulating Microparticles in the Pathogenesis and Early Anticoagulation of Thrombosis in COVID-19 With Kidney Injury.Front Cell Dev Biol. 2022 Jan 18;9:784505. doi: 10.3389/fcell.2021.784505. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35118071 Free PMC article. Review.
-
Review of the mechanism of infection induced cerebral small vessel disease.Front Immunol. 2025 May 26;16:1594891. doi: 10.3389/fimmu.2025.1594891. eCollection 2025. Front Immunol. 2025. PMID: 40491910 Free PMC article. Review.
-
Protection by metformin against severe Covid-19: An in-depth mechanistic analysis.Diabetes Metab. 2022 Jul;48(4):101359. doi: 10.1016/j.diabet.2022.101359. Epub 2022 May 31. Diabetes Metab. 2022. PMID: 35662580 Free PMC article. Review.
-
Molecular Insights into SARS-CoV2-Induced Alterations of the Gut/Brain Axis.Int J Mol Sci. 2021 Sep 28;22(19):10440. doi: 10.3390/ijms221910440. Int J Mol Sci. 2021. PMID: 34638785 Free PMC article.
References
-
- COVID-19 Map (2020). Coronavirus Resource Centre, John Hopkins University (JHU). https://coronavirus.jhu.edu/map.html. Accessed Jan 15, 2021
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

