Metalloenzyme-Inspired Ce-MOF Catalyst for Oxidative Halogenation Reactions
- PMID: 34176269
- PMCID: PMC9131423
- DOI: 10.1021/acsami.1c07496
Metalloenzyme-Inspired Ce-MOF Catalyst for Oxidative Halogenation Reactions
Abstract
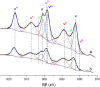
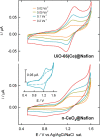
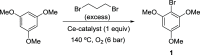
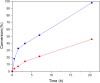
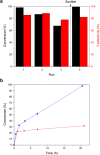
The structure of UiO-66(Ce) is formed by CeO2-x defective nanoclusters connected by terephthalate ligands. The initial presence of accessible Ce3+ sites in the as-synthesized UiO-66(Ce) has been determined by X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared (FTIR)-CO analyses. Moreover, linear scan voltammetric measurements reveal a reversible Ce4+/Ce3+ interconversion within the UiO-66(Ce) material, while nanocrystalline ceria shows an irreversible voltammetric response. This suggests that terephthalic acid ligands facilitate charge transfer between subnanometric metallic nodes, explaining the higher oxidase-like activity of UiO-66(Ce) compared to nanoceria for the mild oxidation of organic dyes under aerobic conditions. Based on these results, we propose the use of Ce-based metal-organic frameworks (MOFs) as efficient catalysts for the halogenation of activated arenes, as 1,3,5-trimethoxybenzene (TMB), using oxygen as a green oxidant. Kinetic studies demonstrate that UiO-66(Ce) is at least three times more active than nanoceria under the same reaction conditions. In addition, the UiO-66(Ce) catalyst shows an excellent stability and can be reused after proper washing treatments. Finally, a general mechanism for the oxidative halogenation reaction is proposed when using Ce-MOF as a catalyst, which mimics the mechanistic pathway described for metalloenzymes. The superb control in the generation of subnanometric CeO2-x defective clusters connected by adequate organic ligands in MOFs offers exciting opportunities in the design of Ce-based redox catalysts.
Keywords: Ce-MOF; ligand-to-metal charge transfer; oxidase activity; oxidative halogenation; subnanometric CeO2−x clusters.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Lu L.; Li Y.; Jiang X. Visible-Light-Promoted Oxidative Halogenation of (Hetero)Arenes. Green Chem. 2020, 22, 5989–5994. 10.1039/D0GC02091E. - DOI
-
- Metal-Catalyzed Cross-Coupling Reactions; Diederich F.; Stang P. J., Eds.; John Wiley & Sons, 2008.

