When the human brain goes diving: using near-infrared spectroscopy to measure cerebral and systemic cardiovascular responses to deep, breath-hold diving in elite freedivers
- PMID: 34176327
- PMCID: PMC8237162
- DOI: 10.1098/rstb.2020.0349
When the human brain goes diving: using near-infrared spectroscopy to measure cerebral and systemic cardiovascular responses to deep, breath-hold diving in elite freedivers
Abstract
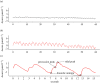
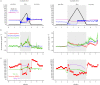
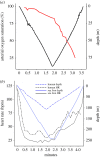
Continuous measurements of haemodynamic and oxygenation changes in free living animals remain elusive. However, developments in biomedical technologies may help to fill this knowledge gap. One such technology is continuous-wave near-infrared spectroscopy (CW-NIRS)-a wearable and non-invasive optical technology. Here, we develop a marinized CW-NIRS system and deploy it on elite competition freedivers to test its capacity to function during deep freediving to 107 m depth. We use the oxyhaemoglobin and deoxyhaemoglobin concentration changes measured with CW-NIRS to monitor cerebral haemodynamic changes and oxygenation, arterial saturation and heart rate. Furthermore, using concentration changes in oxyhaemoglobin engendered by cardiac pulsation, we demonstrate the ability to conduct additional feature exploration of cardiac-dependent haemodynamic changes. Freedivers showed cerebral haemodynamic changes characteristic of apnoeic diving, while some divers also showed considerable elevations in venous blood volumes close to the end of diving. Some freedivers also showed pronounced arterial deoxygenation, the most extreme of which resulted in an arterial saturation of 25%. Freedivers also displayed heart rate changes that were comparable to diving mammals both in magnitude and patterns of change. Finally, changes in cardiac waveform associated with heart rates less than 40 bpm were associated with changes indicative of a reduction in vascular compliance. The success here of CW-NIRS to non-invasively measure a suite of physiological phenomenon in a deep-diving mammal highlights its efficacy as a future physiological monitoring tool for human freedivers as well as free living animals. This article is part of the theme issue 'Measuring physiology in free-living animals (Part II)'.
Keywords: SpO2; breath-hold diving; cererbal oxygenation; diving physiology; freediving; near-infrared spectroscopy.
Figures


Similar articles
-
Cerebral hemodynamic and systemic physiological changes in trained freedivers completing sled-assisted dives to two different depths.Am J Physiol Regul Integr Comp Physiol. 2024 Dec 1;327(6):R553-R567. doi: 10.1152/ajpregu.00085.2024. Epub 2024 Sep 6. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 39241005 Free PMC article.
-
The oxygen-conserving potential of the diving response: A kinetic-based analysis.J Sports Sci. 2017 Apr;35(7):678-687. doi: 10.1080/02640414.2016.1183809. Epub 2016 May 11. J Sports Sci. 2017. PMID: 27167834
-
Case Studies in Physiology: Is blackout in breath-hold diving related to cardiac arrhythmias?J Appl Physiol (1985). 2023 Apr 1;134(4):951-956. doi: 10.1152/japplphysiol.00708.2022. Epub 2023 Feb 24. J Appl Physiol (1985). 2023. PMID: 36825646
-
Breath-Hold Diving.Compr Physiol. 2018 Mar 25;8(2):585-630. doi: 10.1002/cphy.c160008. Compr Physiol. 2018. PMID: 29687909 Review.
-
Physiology, pathophysiology and (mal)adaptations to chronic apnoeic training: a state-of-the-art review.Eur J Appl Physiol. 2021 Jun;121(6):1543-1566. doi: 10.1007/s00421-021-04664-x. Epub 2021 Mar 31. Eur J Appl Physiol. 2021. PMID: 33791844 Free PMC article. Review.
Cited by
-
Dopamine/BDNF loss underscores narcosis cognitive impairment in divers: a proof of concept in a dry condition.Eur J Appl Physiol. 2023 Jan;123(1):143-158. doi: 10.1007/s00421-022-05055-6. Epub 2022 Oct 10. Eur J Appl Physiol. 2023. PMID: 36214902 Free PMC article.
-
Association Between Arterial Oxygen Saturation and Lung Ultrasound B-Lines After Competitive Deep Breath-Hold Diving.Front Physiol. 2021 Aug 4;12:711798. doi: 10.3389/fphys.2021.711798. eCollection 2021. Front Physiol. 2021. PMID: 34421654 Free PMC article.
-
Blood oxygen transport and depletion in diving emperor penguins.J Exp Biol. 2024 Mar 15;227(6):jeb246832. doi: 10.1242/jeb.246832. Epub 2024 Mar 18. J Exp Biol. 2024. PMID: 38390686 Free PMC article.
-
What is physiologging? Introduction to the theme issue, part 2.Philos Trans R Soc Lond B Biol Sci. 2021 Aug 16;376(1831):20210028. doi: 10.1098/rstb.2021.0028. Epub 2021 Jun 28. Philos Trans R Soc Lond B Biol Sci. 2021. PMID: 34176329 Free PMC article.
-
Somatosensory and prefrontal cortex activity relates to emotional outcomes and hair cortisol concentration in chronic postsurgical pain.Sci Rep. 2025 May 10;15(1):16304. doi: 10.1038/s41598-025-00685-0. Sci Rep. 2025. PMID: 40348822 Free PMC article.
References
-
- Scholander PF. 1940. Experimental investigations on the respiratory function in diving birds and mammals. Hvaldradets Skr. 22, 1-31.
-
- Andersson J, Schagatay E. 1998. Arterial oxygen desaturation during apnea in humans. Undersea Hyperbar. Med. 25, 21-25. - PubMed
-
- Daly M. 2011. Interactions between respiration and circulation. In Handbook of physiology: the respiratory system II (eds Cherhiack NS, Widdicombe JG), pp. 529-594. Bethesda, MD: American Physiology Society.
-
- Blix AS, Folkow B. 1983. Cardiovascular adjustments to diving in mammals and birds. In Handbook of physiology. The cardiovascular system. Peripheral circulation and organ blood flow (eds Casson DM, Ronald K), pp. 917-945. Bethesda, MD: American Physiology Society.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

