Protective effects of baicalin on caerulein-induced AR42J pancreatic acinar cells by attenuating oxidative stress through miR-136-5p downregulation
- PMID: 34176350
- PMCID: PMC10305831
- DOI: 10.1177/00368504211026118
Protective effects of baicalin on caerulein-induced AR42J pancreatic acinar cells by attenuating oxidative stress through miR-136-5p downregulation
Abstract
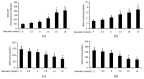
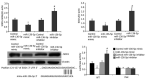
Baicalin, the main active component of Scutellaria baicalensis, has antioxidant and anti-apoptotic effects and is used to treat acute pancreatitis; however, its specific mechanism is unclear. This study aims to determine the protective effect and underlying mechanism of baicalin on AR42J pancreatic acinar cell injury. AR42J acinar cells (caerulein, 10 nmol/L) were induced in vitro to establish a cell model for acute pancreatitis. Cell relative survival was measured by thiazolyl blue tetrazolium bromide, and cell apoptosis and death were examined by flow cytometry. The expression levels of superoxide dismutase1 (SOD1), Bax, survivin, Bcl-2, caspase-3, and caspase-7 proteins were analyzed by Western blot, and those of SOD1 mRNA and miR-136-5p were determined by RT-PCR. The activities of GSH, SOD1, ROS, and MDA were also investigated. Compared with those of the caerulein group, the relative survival rate and activity of AR42J pancreatic acinar cells with different baicalin concentrations were significantly increased (p < 0.05), and the supernatant amylase level was markedly decreased (p < 0.05). In addition, the ROS and MDA activities and mir-136-5p expression were significantly decreased, and the GSH activities and SOD1 gene and protein expression levels were markedly increased (p < 0.05). These results suggest that baicalin reduced the caerulein-induced death of AR42J acinar cells and alleviated the caerulein-induced injury in pancreatic acinar cells by inhibiting oxidative stress. The mechanism may be related to the decreased expression of Mir-136-5p and the increased expression of SOD1 gene and protein.
Keywords: AR42J acinar cells; Baicalin; acute pancreatitis; miR-136-5p; oxidative stress; superoxide dismutase 1.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures











Similar articles
-
Circ_0000284 Promoted Acute Pancreatitis Progression through the Regulation of miR-10a-5p/Wnt/β-Catenin Pathway.Chem Biodivers. 2022 Jun;19(6):e202101006. doi: 10.1002/cbdv.202101006. Epub 2022 May 17. Chem Biodivers. 2022. PMID: 35581162
-
Effect of microRNA-27a-5p on apoptosis and inflammatory response of pancreatic acinar cells in acute pancreatitis by targeting PTEN.J Cell Biochem. 2019 Sep;120(9):15844-15850. doi: 10.1002/jcb.28855. Epub 2019 May 20. J Cell Biochem. 2019. PMID: 31106896
-
Circ_ZFP644 attenuates caerulein-induced inflammatory injury in rat pancreatic acinar cells by modulating miR-106b/Pias3 axis.Exp Mol Pathol. 2021 Aug;121:104644. doi: 10.1016/j.yexmp.2021.104644. Epub 2021 May 1. Exp Mol Pathol. 2021. PMID: 33945806
-
Downregulation of lncRNA NEAT1 Relieves Caerulein-Induced Cell Apoptosis and Inflammatory Injury in AR42J Cells Through Sponging miR-365a-3p in Acute Pancreatitis.Biochem Genet. 2022 Dec;60(6):2286-2298. doi: 10.1007/s10528-022-10219-2. Epub 2022 Mar 24. Biochem Genet. 2022. PMID: 35325441
-
Acinar Cell-Derived Extracellular Vesicle MiRNA-183-5p Aggravates Acute Pancreatitis by Promoting M1 Macrophage Polarization Through Downregulation of FoxO1.Front Immunol. 2022 Jul 13;13:869207. doi: 10.3389/fimmu.2022.869207. eCollection 2022. Front Immunol. 2022. PMID: 35911777 Free PMC article.
Cited by
-
Recent insights into the biological functions of baicalin.EXCLI J. 2022 Aug 1;21:1019-1027. doi: 10.17179/excli2022-5184. eCollection 2022. EXCLI J. 2022. PMID: 36172075 Free PMC article. No abstract available.
-
The Role of MicroRNAs in Pancreatitis Development and Progression.Int J Mol Sci. 2023 Jan 5;24(2):1057. doi: 10.3390/ijms24021057. Int J Mol Sci. 2023. PMID: 36674571 Free PMC article. Review.
-
From micro to macro, nanotechnology demystifies acute pancreatitis: a new generation of treatment options emerges.J Nanobiotechnology. 2025 Jan 29;23(1):57. doi: 10.1186/s12951-025-03106-6. J Nanobiotechnology. 2025. PMID: 39881355 Free PMC article. Review.
-
Targeting PFKFB3 to restore glucose metabolism in acute pancreatitis via nanovesicle delivery.Mol Med. 2025 Jul 5;31(1):253. doi: 10.1186/s10020-025-01261-y. Mol Med. 2025. PMID: 40618046 Free PMC article.
-
Diagnostic and prognostic value of miR-146b-5p in acute pancreatitis.Hereditas. 2025 May 31;162(1):93. doi: 10.1186/s41065-025-00466-9. Hereditas. 2025. PMID: 40450371 Free PMC article.
References
-
- Amas Gómez L, Zubia Olaskoaga F. Results of the modification of an acute pancreatitis management protocol in Intensive Care medicine. Med Intensiva 2019; 43(9): 546–555. - PubMed
-
- Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ 2019; 367: l6227. - PubMed
-
- Ma Z, Song G, Zhao D, et al.. Bone marrow-derived mesenchymal stromal cells ameliorate severe acute pancreatitis in rats via hemeoxygenase-1-mediated anti-oxidant and anti-inflammatory effects. Cytotherapy 2019; 21(2): 162–174. - PubMed
-
- Máté G, Gazdag Z, Mike N, et al.. Regulation of oxidative stress-induced cytotoxic processes of citrinin in the fission yeast Schizosaccharomyces pombe. Toxicon 2014; 90: 155–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

