Nanoparticles prepared from pterostilbene reduce blood glucose and improve diabetes complications
- PMID: 34176494
- PMCID: PMC8237509
- DOI: 10.1186/s12951-021-00928-y
Nanoparticles prepared from pterostilbene reduce blood glucose and improve diabetes complications
Abstract
Background: Diabetes complications are the leading cause of mortality in diabetic patients. The common complications are decline in antioxidant capacity and the onset of micro-inflammation syndrome. At present, glucose-responsive nanoparticles are widely used, as they can release insulin-loaded ultrafine particles intelligently and effectively reduce blood sugar. However, the toxicology of this method has not been fully elucidated. The plant extracts of pterostilbene (PTE) have a wide range of biological applications, such as antioxidation and inflammatory response improvement. Therefore, we have proposed new ideas for the cross application of plant extracts and biomaterials, especially as part of a hypoglycaemic nano-drug delivery system.
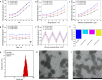
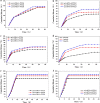
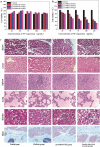
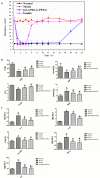
Results: Based on the PTE, we successfully synthesised poly(3-acrylamidophenyl boric acid-b-pterostilbene) (p[AAPBA-b-PTE]) nanoparticles (NPs). The NPs were round in shape and ranged between 150 and 250 nm in size. The NPs possessed good pH and glucose sensitivity. The entrapment efficiency (EE) of insulin-loaded NPs was approximately 56%, and the drug loading (LC) capacity was approximately 13%. The highest release of insulin was 70%, and the highest release of PTE was 85%. Meanwhile, the insulin could undergo self-regulation according to changes in the glucose concentration, thus achieving an effective, sustained release. Both in vivo and in vitro experiments showed that the NPs were safe and nontoxic. Under normal physiological conditions, NPs were completely degraded within 40 days. Fourteen days after mice were injected with p(AAPBA-b-PTE) NPs, there were no obvious abnormalities in the heart, liver, spleen, lung, or kidney. Moreover, NPs effectively reduced blood glucose, improved antioxidant capacity and reversed micro-inflammation in mice.
Conclusions: p(AAPBA-b-PTE) NPs were successfully prepared using PTE as raw material and effectively reduced blood glucose, improved antioxidant capacity and reduced the inflammatory response. This novel preparation can enable new combinations of plant extracts and biomaterials to adiministered through NPs or other dosage forms in order to regulate and treat diseases.
Keywords: 3-acrylamidophenylboronic acid (AAPBA); Diabetes complications; Diabetes mellitus; Insulin delivery; Nano-carrier; Pterostilbene (PTE).
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Glucose-Sensitive Nanoparticles Based On Poly(3-Acrylamidophenylboronic Acid-Block-N-Vinylcaprolactam) For Insulin Delivery.Int J Nanomedicine. 2019 Oct 4;14:8059-8072. doi: 10.2147/IJN.S220936. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31632018 Free PMC article.
-
Glucose- and temperature-sensitive nanoparticles for insulin delivery.Int J Nanomedicine. 2017 May 29;12:4037-4057. doi: 10.2147/IJN.S132984. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28603417 Free PMC article.
-
Phenylboronate-diol crosslinked glycopolymeric nanocarriers for insulin delivery at physiological pH.Soft Matter. 2014 Feb 14;10(6):911-20. doi: 10.1039/c3sm52485j. Soft Matter. 2014. PMID: 24835766
-
Nanoparticle Delivery Systems in the Treatment of Diabetes Complications.Molecules. 2019 Nov 20;24(23):4209. doi: 10.3390/molecules24234209. Molecules. 2019. PMID: 31756981 Free PMC article. Review.
-
Nanomaterials for diabetes: diagnosis, detection and delivery.Nanotechnology. 2024 Jul 11;35(39). doi: 10.1088/1361-6528/ad5db5. Nanotechnology. 2024. PMID: 38990067 Review.
Cited by
-
Amorphous Pterostilbene Delivery Systems Preparation-Innovative Approach to Preparation Optimization.Pharmaceutics. 2023 Apr 13;15(4):1231. doi: 10.3390/pharmaceutics15041231. Pharmaceutics. 2023. PMID: 37111715 Free PMC article.
-
pH-switchable nanozyme cascade catalysis: a strategy for spatial-temporal modulation of pathological wound microenvironment to rescue stalled healing in diabetic ulcer.J Nanobiotechnology. 2022 Jan 4;20(1):12. doi: 10.1186/s12951-021-01215-6. J Nanobiotechnology. 2022. PMID: 34983560 Free PMC article.
-
New Insights into Dietary Pterostilbene: Sources, Metabolism, and Health Promotion Effects.Molecules. 2022 Sep 25;27(19):6316. doi: 10.3390/molecules27196316. Molecules. 2022. PMID: 36234852 Free PMC article. Review.
-
Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects.Int J Nanomedicine. 2023 Sep 5;18:4987-5009. doi: 10.2147/IJN.S420748. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37693885 Free PMC article. Review.
-
Novel glucose-responsive nanoparticles based on p-hydroxyphenethyl anisate and 3-acrylamidophenylboronic acid reduce blood glucose and ameliorate diabetic nephropathy.Mater Today Bio. 2021 Dec 3;13:100181. doi: 10.1016/j.mtbio.2021.100181. eCollection 2022 Jan. Mater Today Bio. 2021. PMID: 34927045 Free PMC article.
References
-
- van der Schaft N, Schoufour JD, Nano J, Kiefte-de Jong JC, Muka T, Sijbrands EJG, Ikram MA, Franco OH, Voortman T. Dietary antioxidant capacity and risk of type 2 diabetes mellitus, prediabetes and insulin resistance: the Rotterdam Study. Eur J Epidemiol. 2019;34(9):853–861. doi: 10.1007/s10654-019-00548-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

