Public access to protocols of contemporary cancer randomized clinical trials
- PMID: 34176506
- PMCID: PMC8237482
- DOI: 10.1186/s13063-021-05382-7
Public access to protocols of contemporary cancer randomized clinical trials
Abstract
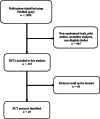
Access to randomized clinical trial (RCT) protocols is necessary for the interpretation and reproducibility of the study results, but protocol availability has been lacking. We determined the prevalence of protocol availability for all published cancer RCTs in January 2020. We found that only 36.1% (48/133) of RCTs had an accessible protocol and only 11.3% of RCTs (15/133) had a publicly accessible protocol that was not behind a paywall. Only 18.0% (24/133) of RCTs were published in conjunction with the protocol on the journal website. In conclusion, few cancer RCTs have an accessible research protocol. Journals should require publication of RCT protocols along with manuscripts to improve research transparency.
Keywords: Access; Cancer; Clinical trials; Protocols.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical