Quality of life and its predictors in adults with tuberous sclerosis complex (TSC): a multicentre cohort study from Germany
- PMID: 34176514
- PMCID: PMC8237479
- DOI: 10.1186/s42466-021-00130-3
Quality of life and its predictors in adults with tuberous sclerosis complex (TSC): a multicentre cohort study from Germany
Abstract
Background: Tuberous sclerosis complex (TSC) is a monogenetic, multisystemic disease characterised by the formation of benign tumours that can affect almost all organs, caused by pathogenic variations in TSC1 or TSC2. In this multicentre study from Germany, we investigated the influence of sociodemographic, clinical, and therapeutic factors on quality of life (QoL) among individuals with TSC.
Methods: We assessed sociodemographic and clinical characteristics and QoL among adults with TSC throughout Germany using a validated, three-month, retrospective questionnaire. We examined predictors of health-related QoL (HRQoL) using multiple linear regression analysis and compared the QoL among patients with TSC with QoL among patients with other chronic neurological disorders.
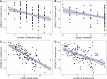
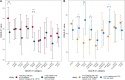
Results: We enrolled 121 adults with TSC (mean age: 31.0 ± 10.5 years; range: 18-61 years, 45.5% [n = 55] women). Unemployment, a higher grade of disability, a higher number of organ manifestations, the presence of neuropsychiatric manifestations or active epilepsy, and a higher burden of therapy-related adverse events were associated with worse QoL, as measured by two QoL instruments (EuroQoL-5 dimensions [EQ-5D] and Quality of Life in Epilepsy Patients [QOLIE-31]). Neuropsychiatric and structural nervous system manifestations, the number of affected organs, and therapy-related adverse events were also associated with higher depression, as measured by the Neurological Disorders Depression Inventory for Epilepsy (NDDI-E). In multiple regression analysis, more severe therapy-related adverse events (large effect, p < 0.001), active epilepsy (large effect, p < 0.001), and neuropsychiatric manifestations (medium effect, p = 0.003) were independently associated with worse HRQoL, explaining 65% of the variance (p < 0.001). The HRQoL among patients with active TSC-associated epilepsy was worse than that among patients with drug-refractory mesial temporal lobe epilepsy (p < 0.001), and the generic QoL among patients with more than three TSC organ manifestations was similar to those of patients with severe migraine and uncontrolled asthma.
Conclusions: Active epilepsy, neuropsychiatric manifestations (such as anxiety and depression), and therapy-related adverse events are important independent predictors of worse quality of life among adults with TSC. Generic quality of life in TSC with several manifestations is similar to uncontrolled severe chronic diseases and significantly negatively correlates with TSC severity.
Trial registration: DRKS, DRKS00016045 . Registered 01 March 2019.
Keywords: EQ-5D; Epilepsy; Organ manifestations; QOLIE-31; Quality of life; Rare disease; Seizure; TSC; Tuberous sclerosis complex; mTOR inhibitor.
Conflict of interest statement
JPZ reports speakers’ honoraria and travel grants from Eisai and Desitin Arzneimittel.
FR reports personal fees from Arvelle Therapeutics, Desitin Arzneimittel, Eisai, GW Pharmaceuticals companies, Novartis, Medtronic, Sandoz, Shire, and UCB and grants from the Detlev-Wrobel-Fonds for Epilepsy Research, the Deutsche Forschungsgemeinschaft, the LOEWE Programme of the State of Hesse, and the European Union.
MS reports personal fees from Novartis and GW Pharmaceuticals companies.
GK reports personal fees from Desitin Arzneimittel, Eisai, GW Pharmaceuticals companies, UCB, Novartis, Takeda, and Zogenix.
TM reports personal fees and grants from Arvelle Therapeutics, Eisai, GW Pharmaceuticals companies, UCB, and Zogenix.
CH reports personal fees from Desitin Arzneimittel, Eisai, GW Pharmaceuticals companies, Novartis, Shire, and Zogenix.
AB reports personal fees from Desitin Arzneimittel GmbH, Eisai GmbH, GW Pharma GmbH, Shire/Takeda GmbH, UCB Pharma GmbH, and ViroPharma GmbH.
KMK reports personal fees from UCB Pharma, Novartis Pharma AG, Eisai, and GW Pharmaceuticals and grants from the federal state Hessen, through the LOEWE program, and from the Canadian Institutes of Health Research.
SM reports grants from Novartis, UCB, Shire, Deutsche Forschungsgemeinschaft, and Epilepsiestiftung Dr. Wolf.
FvP reports personal fees and grants from Bial, Desitin Arzneimittel, Eisai, GW Pharmaceutical companies, Arvelle Therapeutics, Zogenix, and UCB Pharma.
SS-B reports personal fees from Eisai, Desitin Pharma, GW Pharmaceuticals companies, LivaNova, UCB, and Zogenix.
AS reports personal fees and grants from Arvelle Therapeutics, Desitin Arzneimittel, Eisai, GW Pharmaceutical companies, LivaNova, Marinus Pharma, Medtronic, UCB, and Zogenix.
None of the other authors reported any related conflicts of interest.
Figures

Similar articles
-
Health-related quality of life in children and adolescents with tuberous sclerosis complex and their caregivers: A multicentre cohort study from Germany.Eur J Paediatr Neurol. 2021 Nov;35:111-122. doi: 10.1016/j.ejpn.2021.10.003. Epub 2021 Oct 7. Eur J Paediatr Neurol. 2021. PMID: 34673401
-
Direct and indirect costs and cost-driving factors in adults with tuberous sclerosis complex: a multicenter cohort study and a review of the literature.Orphanet J Rare Dis. 2021 Jun 2;16(1):250. doi: 10.1186/s13023-021-01838-w. Orphanet J Rare Dis. 2021. PMID: 34078440 Free PMC article. Review.
-
Direct and indirect costs and cost-driving factors of Tuberous sclerosis complex in children, adolescents, and caregivers: a multicenter cohort study.Orphanet J Rare Dis. 2021 Jun 21;16(1):282. doi: 10.1186/s13023-021-01899-x. Orphanet J Rare Dis. 2021. PMID: 34154622 Free PMC article.
-
Neuropsychiatric profile in tuberous sclerosis complex patients with epilepsy.Front Pediatr. 2025 Jan 15;12:1436061. doi: 10.3389/fped.2024.1436061. eCollection 2024. Front Pediatr. 2025. PMID: 39882213 Free PMC article.
-
Review of the treatment options for epilepsy in tuberous sclerosis complex: towards precision medicine.Ther Adv Neurol Disord. 2021 Jul 17;14:17562864211031100. doi: 10.1177/17562864211031100. eCollection 2021. Ther Adv Neurol Disord. 2021. PMID: 34349839 Free PMC article. Review.
Cited by
-
Exploring the relationships between composite scores of disease severity, seizure-freedom and quality of life in Dravet syndrome.Neurol Res Pract. 2022 Jun 6;4(1):22. doi: 10.1186/s42466-022-00186-9. Neurol Res Pract. 2022. PMID: 35659154 Free PMC article.
-
The research landscape of tuberous sclerosis complex-associated neuropsychiatric disorders (TAND)-a comprehensive scoping review.J Neurodev Disord. 2022 Feb 13;14(1):13. doi: 10.1186/s11689-022-09423-3. J Neurodev Disord. 2022. PMID: 35151277 Free PMC article.
-
Psychobehavioural and Cognitive Adverse Events of Anti-Seizure Medications for the Treatment of Developmental and Epileptic Encephalopathies.CNS Drugs. 2022 Oct;36(10):1079-1111. doi: 10.1007/s40263-022-00955-9. Epub 2022 Oct 4. CNS Drugs. 2022. PMID: 36194365 Free PMC article. Review.
-
Understanding the impact of tuberous sclerosis complex: development and validation of the TSC-PROM.BMC Med. 2023 Aug 8;21(1):298. doi: 10.1186/s12916-023-03012-4. BMC Med. 2023. PMID: 37553648 Free PMC article.
-
Diagnosis and Management of Tuberous Sclerosis Complex in a Resource-Limited Setting-A Case Report of a 14-Year-Old Female Zambian Adolescent.Clin Med Insights Case Rep. 2025 Feb 18;18:11795476251321268. doi: 10.1177/11795476251321268. eCollection 2025. Clin Med Insights Case Rep. 2025. PMID: 39974288 Free PMC article.
References
-
- Ebrahimi-Fakhari D, Mann LL, Poryo M, Graf N, von Kries R, Heinrich B, Ebrahimi-Fakhari D, Flotats-Bastardas M, Gortner L, Zemlin M, Meyer S. Incidence of tuberous sclerosis and age at first diagnosis: new data and emerging trends from a national, prospective surveillance study. Orphanet Journal of Rare Diseases. 2018;13(1):117. doi: 10.1186/s13023-018-0870-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
