Effectiveness of two intramuscular combined vaccines for the control of Mycoplasma hyopneumoniae and porcine circovirus type 2 in growing pigs: a randomized field trial
- PMID: 34176520
- PMCID: PMC8237417
- DOI: 10.1186/s40813-021-00220-3
Effectiveness of two intramuscular combined vaccines for the control of Mycoplasma hyopneumoniae and porcine circovirus type 2 in growing pigs: a randomized field trial
Abstract
Background: Mycoplasma hyopneumoniae and Porcine circovirus type 2 are two economically important pathogens affecting growing pigs. Control and prevention of both diseases can be accomplished by vaccination, together with biosecurity and good management practices. Many commercial vaccines are available. The aim of this study was to assess the efficacy of Hyogen® and Circovac® administered mixed at weaning and to compare this protocol with a competitor ready-to-use (RTU) vaccine.
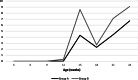
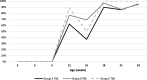
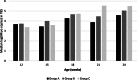
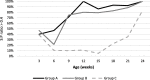
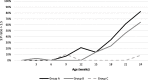
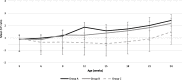
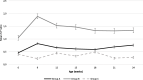
Case presentation: A randomised field trial was designed in a commercial farrow-to-finish farm located in France. A total of 641 pigs born from 54 different sows were included in this study. Piglets at weaning were allocated into three groups: the first one vaccinated with Hyogen® and Circovac® combined (group A), the second one vaccinated with a competitor RTU vaccine (group B) and the last one unvaccinated. Only minor local reactions for both vaccination groups could be observed which revealed a good safety of both protocols. Both vaccination schemes in this trial didn't improve wean-to-slaughter growth performances but significantly reduced lung lesions, lung fissures and pleurisy at slaughter, produced a seroconversion for both M. hyopneumoniae and PCV-2 and significantly reduced the PCV-2 viral load in blood. When we compared groups A and B, we observed no significant differences in growth performances, mortality, clinical signs, percentages of affected lungs at slaughter, lung fissures and pleurisy, and no difference in pathogens detection. However, two statistical differences were observed between both vaccines: the mean lung lesion score and the percentage of extensive lung lesions were lower in group A. This is consistent with lower M. hyopneumoniae loads in the lower respiratory tract in pigs from group A but this difference was not statistically significant.
Conclusions: Results reported in this case study must be considered with caution since it was done in only one farm. In this trial, Hyogen® and Circovac® mixed together under field conditions offered a successful protection of growing pigs and significantly decreased the extension of lung lesions during a natural field challenge when compared with a competitor RTU vaccine.
Keywords: Mycoplasma hyopneumoniae; Porcine circovirus 2; Serology; Vaccine; qPCR.
Conflict of interest statement
SB, MC, NC and RK are employed by Ceva Animal Health France (10, avenue de la Ballastière, 33500 Libourne, France) and were not involved in analysis and interpretation of the data. SB, NC and RK assisted AL and GB for the study design. SB and MC provided assistance for samples and data collection.
Figures
Similar articles
-
A field efficacy and safety trial in the Netherlands in pigs vaccinated at 3 weeks of age with a ready-to-use porcine circovirus type 2 and Mycoplasma hyopneumoniae combined vaccine.Porcine Health Manag. 2017 Nov 9;3:23. doi: 10.1186/s40813-017-0070-5. eCollection 2017. Porcine Health Manag. 2017. PMID: 29152324 Free PMC article.
-
Field evaluation of piglet vaccination with a Mycoplasma hyopneumoniae bacterin as compared to a ready-to-use product including porcine circovirus 2 and M. hyopneumoniae in a conventional French farrow-to-finish farm.Porcine Health Manag. 2018 Jan 18;4:4. doi: 10.1186/s40813-017-0077-y. eCollection 2018. Porcine Health Manag. 2018. PMID: 29375890 Free PMC article.
-
Comparison of effects of a single dose of MHYOSPHERE® PCV ID with three commercial porcine vaccine associations against Mycoplasma hyopneumoniae (Mhyo) and porcine circovirus type 2 (PCV2) on piglet growth during the nursery period under field conditions.Vet Res Commun. 2022 Dec;46(4):1167-1173. doi: 10.1007/s11259-022-09971-y. Epub 2022 Jul 13. Vet Res Commun. 2022. PMID: 35829861 Free PMC article.
-
Comparison of Mycoplasma hyopneumoniae and porcine circovirus 2 commercial vaccines efficacy when applied separate or combined under experimental conditions.Porcine Health Manag. 2020 May 4;6:11. doi: 10.1186/s40813-020-00148-0. eCollection 2020. Porcine Health Manag. 2020. PMID: 32391165 Free PMC article.
-
Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae.Vet J. 2016 Jun;212:1-6. doi: 10.1016/j.tvjl.2015.10.030. Epub 2015 Oct 23. Vet J. 2016. PMID: 27256017 Review.
Cited by
-
Chitosan-Based Nanomaterial as Immune Adjuvant and Delivery Carrier for Vaccines.Vaccines (Basel). 2022 Nov 11;10(11):1906. doi: 10.3390/vaccines10111906. Vaccines (Basel). 2022. PMID: 36423002 Free PMC article. Review.
-
Porcine respiratory disease complex: Dynamics of polymicrobial infections and management strategies after the introduction of the African swine fever.Front Vet Sci. 2022 Nov 25;9:1048861. doi: 10.3389/fvets.2022.1048861. eCollection 2022. Front Vet Sci. 2022. PMID: 36504860 Free PMC article. Review.
References
-
- Baccaro MR, Hirose F, Umehara O, Gonçalves LCB, Doto DS, Paixão R, Shinya LT, Moreno AM. Comparative efficacy of two single-dose bacterins in the control of mycoplasma hyopneumoniae in swine raised under commercial conditions in Brazil. Vet J. 2006;172(3):526–531. doi: 10.1016/j.tvjl.2005.07.012. - DOI - PubMed
LinkOut - more resources
Full Text Sources

