Organic Polymer Hosts for Triplet-Triplet Annihilation Upconversion Systems
- PMID: 34176961
- PMCID: PMC8223484
- DOI: 10.1021/acs.macromol.1c00133
Organic Polymer Hosts for Triplet-Triplet Annihilation Upconversion Systems
Abstract
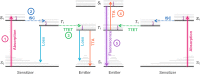
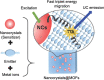
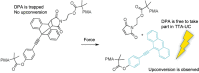
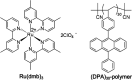
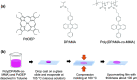
Triplet-triplet annihilation upconversion (TTA-UC) is a process by which a lower energy photon can be upconverted to a higher energy state. The incorporation of TTA-UC materials into solid-state hosts has enabled advances in solar energy and many other applications. The choice of host system is, however, far from trivial and often calls for a careful compromise between characteristics such as high molecular mobility, low oxygen diffusion, and high material stability, factors that often contradict one another. Here, we evaluate these challenges in the context of the state-of-the-art of primarily polymer hosts and the advantages they hold in terms of material selection and tunability of their diffusion or mechanical or thermal properties. We encourage more collaborative research between polymer scientists and photophysicists in order to further optimize the current systems and outline our thoughts for the future direction of the field.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



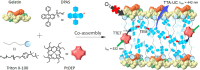



Similar articles
-
Ambient solid-state triplet-triplet annihilation upconversion in ureasil organic-inorganic hybrid hosts.J Mater Chem C Mater. 2024 Apr 15;12(17):6310-6318. doi: 10.1039/d4tc00562g. eCollection 2024 May 2. J Mater Chem C Mater. 2024. PMID: 38707254 Free PMC article.
-
Enhancing Triplet-Triplet Annihilation Upconversion: From Molecular Design to Present Applications.Acc Chem Res. 2022 Sep 20;55(18):2604-2615. doi: 10.1021/acs.accounts.2c00307. Epub 2022 Sep 8. Acc Chem Res. 2022. PMID: 36074952
-
Photon upconversion: from two-photon absorption (TPA) to triplet-triplet annihilation (TTA).Phys Chem Chem Phys. 2016 Apr 28;18(16):10818-35. doi: 10.1039/c5cp07296d. Phys Chem Chem Phys. 2016. PMID: 26843136
-
Designing next generation of photon upconversion: Recent advances in organic triplet-triplet annihilation upconversion nanoparticles.Biomaterials. 2019 May;201:77-86. doi: 10.1016/j.biomaterials.2019.02.008. Epub 2019 Feb 12. Biomaterials. 2019. PMID: 30802685 Free PMC article. Review.
-
Nanoengineering Triplet-Triplet Annihilation Upconversion: From Materials to Real-World Applications.ACS Nano. 2023 Feb 28;17(4):3259-3288. doi: 10.1021/acsnano.3c00543. Epub 2023 Feb 17. ACS Nano. 2023. PMID: 36800310 Review.
Cited by
-
Microstructure-driven annihilation effects and dispersive excited state dynamics in solid-state films of a model sensitizer for photon energy up-conversion applications.Chem Sci. 2023 Jan 25;14(8):2009-2023. doi: 10.1039/d2sc06426j. eCollection 2023 Feb 22. Chem Sci. 2023. PMID: 36845913 Free PMC article.
-
Plasmonic Metal Nanostructures Meet Triplet-Triplet Annihilation-Based Photon Upconversion Systems: Performance Improvements and Application Trends.Nanomaterials (Basel). 2023 May 5;13(9):1559. doi: 10.3390/nano13091559. Nanomaterials (Basel). 2023. PMID: 37177104 Free PMC article. Review.
-
The Rise and Current Status of Polaritonic Photochemistry and Photophysics.Chem Rev. 2023 Sep 27;123(18):10877-10919. doi: 10.1021/acs.chemrev.2c00895. Epub 2023 Sep 8. Chem Rev. 2023. PMID: 37683254 Free PMC article. Review.
-
Ambient solid-state triplet-triplet annihilation upconversion in ureasil organic-inorganic hybrid hosts.J Mater Chem C Mater. 2024 Apr 15;12(17):6310-6318. doi: 10.1039/d4tc00562g. eCollection 2024 May 2. J Mater Chem C Mater. 2024. PMID: 38707254 Free PMC article.
-
Exploring the Versatile Uses of Triplet States: Working Principles, Limitations, and Recent Progress in Phosphorescence, TADF, and TTA.ACS Appl Opt Mater. 2024 Apr 19;2(12):2476-2500. doi: 10.1021/acsaom.4c00041. eCollection 2024 Dec 27. ACS Appl Opt Mater. 2024. PMID: 39744471 Free PMC article. Review.
References
-
- Parker C. A.; Hatchard C. G. Delayed Fluorescence from Solutions of Anthracene and Phenanthrene. Proc. Chem. Soc. 1962, 269 (1339), 574–584. 10.1098/rspa.1962.0197. - DOI
-
- Monguzzi A.; Mauri M.; Frigoli M.; Pedrini J.; Simonutti R.; Larpent C.; Vaccaro G.; Sassi M.; Meinardi F. Unraveling Triplet Excitons Photophysics in Hyper-Cross-Linked Polymeric Nanoparticles: Toward the Next Generation of Solid-State Upconverting Materials. J. Phys. Chem. Lett. 2016, 7 (14), 2779–2785. 10.1021/acs.jpclett.6b01115. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
