The Unfolded Protein Response in Immune Cells as an Emerging Regulator of Neuroinflammation
- PMID: 34177557
- PMCID: PMC8226365
- DOI: 10.3389/fnagi.2021.682633
The Unfolded Protein Response in Immune Cells as an Emerging Regulator of Neuroinflammation
Abstract
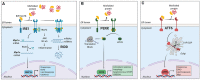
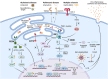
Immune surveillance is an essential process that safeguards the homeostasis of a healthy brain. Among the increasing diversity of immune cells present in the central nervous system (CNS), microglia have emerged as a prominent leukocyte subset with key roles in the support of brain function and in the control of neuroinflammation. In fact, impaired microglial function is associated with the development of neurodegenerative diseases, including Alzheimer's disease (AD) and Parkinson's disease (PD). Interestingly, these pathologies are also typified by protein aggregation and proteostasis dysfunction at the level of the endoplasmic reticulum (ER). These processes trigger activation of the unfolded protein response (UPR), which is a conserved signaling network that maintains the fidelity of the cellular proteome. Remarkably, beyond its role in protein folding, the UPR has also emerged as a key regulator of the development and function of immune cells. However, despite this evidence, the contribution of the UPR to immune cell homeostasis, immune surveillance, and neuro-inflammatory processes remains largely unexplored. In this review, we discuss the potential contribution of the UPR in brain-associated immune cells in the context of neurodegenerative diseases.
Keywords: ER stress; UPR; immune system; inflammation; microglia; neurodegeneration; neuroinflammation; protein misfolding.
Copyright © 2021 Fernández, Geisse, Bernales, Lira and Osorio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

