Latamoxef for Neonates With Early-Onset Neonatal Sepsis: A Study Protocol for a Randomized Controlled Trial
- PMID: 34177569
- PMCID: PMC8220210
- DOI: 10.3389/fphar.2021.635517
Latamoxef for Neonates With Early-Onset Neonatal Sepsis: A Study Protocol for a Randomized Controlled Trial
Abstract
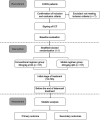
Early-onset neonatal sepsis (EONS), a bacterial infection that occurs within 72 h after birth, is associated with high likelihood of neonatal mortality. Latamoxef, a semi-synthetic oxacephem antibiotic developed in 1980s, has been brought back into empirical EONS treatment in recent years. In the preliminary work, we established a population pharmacokinetics (PPK) model for latamoxef in Chinese neonates. Moreover, in order to better guide clinical treatment, we conducted dose simulation and found that ascending administration frequency could improve the target rate of 70% of patients having a free antimicrobial drug concentration exceeding the MIC during 70% of the dosing interval (70% fT > MIC). Accordingly, this study is aimed to compare the 70% fT > MIC, efficacy and safety between conventional regimen and PPK model regimen for rational use of latamoxef in EONS treatment. A single-blind, multicenter randomized controlled trial (RCT) for latamoxef will be conducted in Chinese EONS patients. Neonates (≤3 days of age, expected number = 114) admitted to the hospital with the diagnosis of EONS and fulfilling inclusion and exclusion criteria will be randomized (ratio of 1:1) to either a conventional regimen (30 mg/kg q12h) or model regimen (20 mg/kg q8h) latamoxef treatment group for at least 3 days. Primary outcome measure will be 70% fT > MIC and secondary outcome indicators will be the latamoxef treatment failure, duration of antibiotic therapy, changes of white blood cell count (WBC), C-reactive protein (CRP) and procalcitonin (PCT), blood culture results during administration and incidence of adverse event (AE)s. Assessments will be made at baseline, initial stage of latamoxef treatment (18-72 h) and before the end of latamoxef treatment. Ethical approval of our clinical trial has been granted by the ethics committee of the Beijing Children's Hospital (ID: 2020-13-1). Written informed consent will be obtained from the parents of the participants. This trial is registered in the Chinese Clinical Trial Registry (ChiCTR 2000040064).It is hoped that our study will provide a clinical basis for the rational clinical use of latamoxef in EONS treatment.
Keywords: early-onset neonatal sepsis; latamoxef; neonate; randomized controlled trial; study protocol.
Copyright © 2021 Qi, Wu, Liu, Kou, Wang, Peng, Chen, Cui, Wang, Li, Zhao and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cui Lan-Qing L. Y. L. Y. (2016). Antimicrobial Susceptibility Surveillance of Moxalactam in China from 2004 to 2014. Chin. J. Clin. Pharmacol. 9, 813–817. 10.13699/j.cnki.1001—6821.2016.09.0015 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous