Exosomes in Intestinal Inflammation
- PMID: 34177577
- PMCID: PMC8220320
- DOI: 10.3389/fphar.2021.658505
Exosomes in Intestinal Inflammation
Abstract
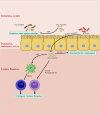
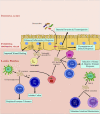
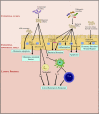
Exosomes are 30-150 nm sized vesicles released by a variety of cells, and are found in most physiological compartments (feces, blood, urine, saliva, breast milk). They can contain different cargo, including nucleic acids, proteins and lipids. In Inflammatory Bowel Disease (IBD), a distinct exosome profile can be detected in blood and fecal samples. In addition, circulating exosomes can carry targets on their surface for monoclonal antibodies used as IBD therapy. This review aims to understand the exosome profile in humans and other mammals, the cargo contained in them, the effect of exosomes on the gut, and the application of exosomes in IBD therapy.
Keywords: IBD; colitis; exosomes; extracellular vesicles; inflammation.
Copyright © 2021 Ayyar and Moss.
Conflict of interest statement
Author ACM has received grant support from Pfizer inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alvarez C.-S., Badia J., Bosch M., Giménez R., Baldomà L. (2016). Outer Membrane Vesicles and Soluble Factors Released by Probiotic escherichia Coli Nissle 1917 and Commensal ECOR63 Enhance Barrier Function by Regulating Expression of Tight junction Proteins in Intestinal Epithelial Cells. Front. Microbiol. 7 (DEC), 1–14. 10.3389/fmicb.2016.01981 - DOI - PMC - PubMed
-
- André F., Chaput N., Schartz N. E. C., Flament C., Aubert N., Bernard J., et al. (2004). Exosomes as Potent Cell-free Peptide-Based Vaccine. I. Dendritic Cell-Derived Exosomes Transfer Functional MHC Class I/Peptide Complexes to Dendritic Cells. J. Immunol. 172 (4), 2126–2136. 10.4049/jimmunol.172.4.2126 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

