Angelicin Alleviates Post-Trauma Osteoarthritis Progression by Regulating Macrophage Polarization via STAT3 Signaling Pathway
- PMID: 34177582
- PMCID: PMC8223070
- DOI: 10.3389/fphar.2021.669213
Angelicin Alleviates Post-Trauma Osteoarthritis Progression by Regulating Macrophage Polarization via STAT3 Signaling Pathway
Abstract
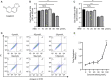
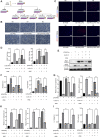
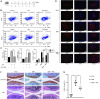
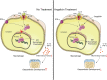
Post-trauma osteoarthritis (PTOA) is the most common articular disease characterized by degeneration and destruction of articular cartilage (Bultink and Lems, Curr. Rheumatol Rep., 2013, 15, 328). Inflammatory response of local joint tissue induced by trauma is the most critical factor accelerating osteoarthritis (OA) progression (Sharma et al., 2019; Osteoarthritis. Cartilage, 28, 658-668). M1/M2 macrophages polarization and repolarization participates in local inflammation, which plays a major role in the progression of OA (Zhang et al., 2018; Ann. Rheum. Dis., 77, 1524-1534). The regulating effect of macrophage polarization has been reported as a potential therapy to alleviate OA progression. Synovitis induced by polarized macrophages could profoundly affect the chondrocyte and cartilage matrix (Zhang et al., 2018; Ann. Rheum. Dis., 77, 1524-1534). Generally, anti-inflammatory medications widely used in clinical practice have serious side effects. Therefore, we focus on exploring a new therapeutic strategy with fewer side effects to alleviate the synovitis. Angelicin (ANG) is traditional medicine used in various folk medicine. Previous studies have revealed that angelicin has an inhibitory effect on inflammation (Wei et al., 2016; Inflammation, 39, 1876-1882), tumor growth (Li et al., 2016; Oncology reports, 36, 3,504-3,512; Wang et al., 2017; Molecular Medicine Reports, 16, 5441-5449), DNA damage (Li et al., 2019; Exp. Ther. Med., 18, 1899-1906), and virus proliferation (Li et al., 2018; Front. Cell. Infect. Microbiol., 8, 178). But its specific effects on influencing the process of OA were rarely reported. In this study, the molecular mechanism of angelicin in vivo and in vitro was clearly investigated. Results showed that angelicin could regulate the M1/M2 ratio and function and alleviate the development of PTOA in the meanwhile. Bone marrow monocytes were isolated and induced by macrophage colony-stimulating factor (M-CSF), lipopolysaccharide (LPS) and interferon (IFN)-γ for M1 polarization and interleukin (IL)-4/IL-13 for M2 polarization. Subsequently, repolarization intervention was performed. The results indicate that angelicin can repolarize M1 toward M2 macrophages by upregulating the expression of CD9. Besides, angelicin can also protect and maintain M2 polarization in the presence of LPS/IFN-γ, and subsequently downregulate the expression of inflammatory mediators such as IL-1β and TNF-α. Mechanistically, angelicin can activate the p-STAT3/STAT3 pathway by conducting CD9/gp130 to repolarize toward M2 macrophages. These results suggest angelicin can alleviate the progression of OA by regulating M1/M2 polarization via the STAT3/p-STAT3 pathway. Therefore, angelicin may have a promising application and potential therapeutic value in OA clinical treatment.
Keywords: angelicin; inflammation; macrophage; osteoarthritis; polarization.
Copyright © 2021 Tian, Zeng, Zhao and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
S-propargyl-cysteine attenuates temporomandibular joint osteoarthritis by regulating macrophage polarization via Inhibition of JAK/STAT signaling.Mol Med. 2025 Apr 7;31(1):128. doi: 10.1186/s10020-025-01186-6. Mol Med. 2025. PMID: 40197110 Free PMC article.
-
Alpha defensin-1 attenuates surgically induced osteoarthritis in association with promoting M1 to M2 macrophage polarization.Osteoarthritis Cartilage. 2021 Jul;29(7):1048-1059. doi: 10.1016/j.joca.2021.04.006. Epub 2021 Apr 21. Osteoarthritis Cartilage. 2021. PMID: 33892137
-
Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages.Free Radic Biol Med. 2019 Dec;145:146-160. doi: 10.1016/j.freeradbiomed.2019.09.024. Epub 2019 Sep 21. Free Radic Biol Med. 2019. PMID: 31550528
-
Macrophage: A Potential Target on Cartilage Regeneration.Front Immunol. 2020 Feb 11;11:111. doi: 10.3389/fimmu.2020.00111. eCollection 2020. Front Immunol. 2020. PMID: 32117263 Free PMC article. Review.
-
Targeting macrophage polarization as a promising therapeutic strategy for the treatment of osteoarthritis.Int Immunopharmacol. 2023 Mar;116:109790. doi: 10.1016/j.intimp.2023.109790. Epub 2023 Feb 1. Int Immunopharmacol. 2023. PMID: 36736223 Review.
Cited by
-
κ-Opioid receptor activation attenuates osteoarthritis synovitis by regulating macrophage polarization through the NF-κB pathway.Acta Biochim Biophys Sin (Shanghai). 2024 Jan 25;56(1):82-95. doi: 10.3724/abbs.2023223. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38013468 Free PMC article.
-
Stat3 Signaling Pathway: A Future Therapeutic Target for Bone-Related Diseases.Front Pharmacol. 2022 Apr 25;13:897539. doi: 10.3389/fphar.2022.897539. eCollection 2022. Front Pharmacol. 2022. PMID: 35548357 Free PMC article. Review.
-
A bibliometrics analysis and visualization of osteoimmunology on osteoarthritis studies.Am J Transl Res. 2022 Jun 15;14(6):4260-4277. eCollection 2022. Am J Transl Res. 2022. PMID: 35836890 Free PMC article.
-
N1-Methyladenosine (m1A) Regulation Associated With the Pathogenesis of Abdominal Aortic Aneurysm Through YTHDF3 Modulating Macrophage Polarization.Front Cardiovasc Med. 2022 May 10;9:883155. doi: 10.3389/fcvm.2022.883155. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35620523 Free PMC article.
-
Exosomes derived from hypoxia-preconditioned M2 macrophages alleviate degeneration in knee osteoarthritis through the miR‑124‑3p/STAT3 axis.J Transl Med. 2025 Jul 10;23(1):772. doi: 10.1186/s12967-025-06808-5. J Transl Med. 2025. PMID: 40640893 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

