Chlorogenic Acid Ameliorates Damage Induced by Fluorene-9-Bisphenol in Porcine Sertoli Cells
- PMID: 34177588
- PMCID: PMC8219976
- DOI: 10.3389/fphar.2021.678772
Chlorogenic Acid Ameliorates Damage Induced by Fluorene-9-Bisphenol in Porcine Sertoli Cells
Abstract
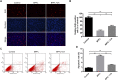
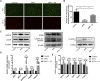
4,4'-(9-Fluorenylidene) diphenol (BPFL, also known as BHPF and fluorene-9-bisphenol) is a novel bisphenol A substitute that is used in the plastics industry as an organic synthesis intermediate and is a potential endocrine disruptor. However, the deleterious effects of BPFL on porcine Sertoli cells (SCs) and the possible underlying mechanisms are still unclear. Chlorogenic acid (CA) is a free radical scavenger in the cellular antioxidant system that prevents oxidative damage and apoptosis. In the present research, we found that BPFL induced impairments in porcine SCs in a dose-dependent manner and that CA protected porcine SCs against BPFL exposure-induced impairments. Cell viability, proliferation and apoptosis assay results revealed that BPFL exposure could inhibit porcine SC proliferation and induce apoptosis, while CA supplementation ameliorated the effects of BPFL. Further analysis revealed that BPFL exposure induced oxidative stress, mitochondrial membrane potential dysfunction and DNA damage accumulation. Transcriptome analysis and further real-time quantitative PCR and Western blot results showed that BPFL exposure induced endoplasmic reticulum stress and apoptosis. Supplementation with CA dramatically ameliorated these phenotypes in BPFL-exposed porcine SCs. Overall, the present research reveals the possible underlying mechanisms by which BPFL exposure induced impairments and CA supplementation protected against these impairments in porcine SCs.
Keywords: BPFL; ameliorates; chlorogenic acid; impairments; porcine sertoli cells.
Copyright © 2021 Zhang, Sun, Wang, Liu, Li, Qi, Zhang, Bai and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Melatonin ameliorates cypermethrin-induced impairments by regulating oxidative stress, DNA damage and apoptosis in porcine Sertoli cells.Theriogenology. 2021 Jun;167:67-76. doi: 10.1016/j.theriogenology.2021.03.011. Epub 2021 Mar 19. Theriogenology. 2021. PMID: 33774368
-
Glycine alleviates fluoride-induced oxidative stress, apoptosis and senescence in a porcine testicular Sertoli cell line.Reprod Domest Anim. 2021 Jun;56(6):884-896. doi: 10.1111/rda.13930. Epub 2021 Mar 25. Reprod Domest Anim. 2021. PMID: 33738852
-
Are BPA-free plastics safe for aquatic life? - Fluorene-9-bisphenol induced thyroid-disrupting effects and histopathological alterations in adult zebrafish (Danio rerio).Comp Biochem Physiol C Toxicol Pharmacol. 2022 Oct;260:109419. doi: 10.1016/j.cbpc.2022.109419. Epub 2022 Jul 25. Comp Biochem Physiol C Toxicol Pharmacol. 2022. PMID: 35902060
-
Effects of Acute Fluorene-9-Bisphenol Exposure on Mouse Oocyte in vitro Maturation and Its Possible Mechanisms.Environ Mol Mutagen. 2019 Apr;60(3):243-253. doi: 10.1002/em.22258. Epub 2018 Nov 30. Environ Mol Mutagen. 2019. PMID: 30499614
-
Fluorene-9-bisphenol exposure induces cytotoxicity in mouse oocytes and causes ovarian damage.Ecotoxicol Environ Saf. 2019 Sep 30;180:168-178. doi: 10.1016/j.ecoenv.2019.05.019. Epub 2019 May 10. Ecotoxicol Environ Saf. 2019. PMID: 31082581
Cited by
-
Chlorogenic acid attenuates liver apoptosis and inflammation in endoplasmic reticulum stress-induced mice.Iran J Basic Med Sci. 2023 Apr;26(4):478-485. doi: 10.22038/IJBMS.2023.66827.14659. Iran J Basic Med Sci. 2023. PMID: 37009010 Free PMC article.
-
Ficus lindsayana Leaf Extract Protects C2C12 Mouse Myoblasts Against the Suppressive Effects of Bisphenol-A on Myogenic Differentiation.Int J Mol Sci. 2025 Jan 8;26(2):476. doi: 10.3390/ijms26020476. Int J Mol Sci. 2025. PMID: 39859191 Free PMC article.
-
Protocol for CRISPR-Cas9 genome editing of a swine cell line via electroporation.STAR Protoc. 2024 Dec 20;5(4):103385. doi: 10.1016/j.xpro.2024.103385. Epub 2024 Oct 10. STAR Protoc. 2024. PMID: 39392744 Free PMC article.
-
Reproductive toxicity of combined effects of endocrine disruptors on human reproduction.Front Cell Dev Biol. 2023 May 12;11:1162015. doi: 10.3389/fcell.2023.1162015. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37250900 Free PMC article. Review.
-
Integrated Metabolomic and Transcriptomic Analysis Revealed the Mechanism of BHPF Exposure in Endometrium.Toxics. 2025 Jan 27;13(2):100. doi: 10.3390/toxics13020100. Toxics. 2025. PMID: 39997915 Free PMC article.
References
LinkOut - more resources
Full Text Sources

