Rifampicin Induces Gene, Protein, and Activity of P-Glycoprotein (ABCB1) in Human Precision-Cut Intestinal Slices
- PMID: 34177592
- PMCID: PMC8220149
- DOI: 10.3389/fphar.2021.684156
Rifampicin Induces Gene, Protein, and Activity of P-Glycoprotein (ABCB1) in Human Precision-Cut Intestinal Slices
Abstract
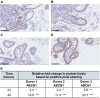
P-glycoprotein (ABCB1), an ATP-binding cassette efflux transporter, limits intestinal absorption of its substrates and is a common site of drug-drug interactions. Drug-mediated induction of intestinal ABCB1 is a clinically relevant phenomenon associated with significantly decreased drug bioavailability. Currently, there are no well-established human models for evaluating its induction, so drug regulatory authorities provide no recommendations for in vitro/ex vivo testing drugs' ABCB1-inducing activity. Human precision-cut intestinal slices (hPCISs) contain cells in their natural environment and express physiological levels of nuclear factors required for ABCB1 induction. We found that hPCISs incubated in William's Medium E for 48 h maintained intact morphology, ATP content, and ABCB1 efflux activity. Here, we asked whether rifampicin (a model ligand of pregnane X receptor, PXR), at 30 μM, induces functional expression of ABCB1 in hPCISs over 24- and 48-h incubation (the time to allow complete induction to occur). Rifampicin significantly increased gene expression, protein levels, and efflux activity of ABCB1. Moreover, we described dynamic changes in ABCB1 transcript levels in hPCISs over 48 h incubation. We also observed that peaks of induction are achieved among donors at different times, and the extent of ABCB1 gene induction is proportional to PXR mRNA levels in the intestine. In conclusion, we showed that hPCISs incubated in conditions comparable to those used for inhibition studies can be used to evaluate drugs' ABCB1-inducing potency in the human intestine. Thus, hPCISs may be valuable experimental tools that can be prospectively used in complex experimental evaluation of drug-drug interactions.
Keywords: P-glycoprotein (ABCB1 protein); absorption; human precision-cut intestinal slices; induction; pregnane X receptor; rifampicin.
Copyright © 2021 Martinec, Biel, de Graaf, Huliciak, de Jong, Staud, Cecka, Olinga, Vokral and Cerveny.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Evaluation of the Potency of Anti-HIV and Anti-HCV Drugs to Inhibit P-Glycoprotein Mediated Efflux of Digoxin in Caco-2 Cell Line and Human Precision-Cut Intestinal Slices.Pharmaceuticals (Basel). 2022 Feb 18;15(2):242. doi: 10.3390/ph15020242. Pharmaceuticals (Basel). 2022. PMID: 35215354 Free PMC article.
-
Anti-HIV and Anti-Hepatitis C Virus Drugs Inhibit P-Glycoprotein Efflux Activity in Caco-2 Cells and Precision-Cut Rat and Human Intestinal Slices.Antimicrob Agents Chemother. 2019 Oct 22;63(11):e00910-19. doi: 10.1128/AAC.00910-19. Print 2019 Nov. Antimicrob Agents Chemother. 2019. PMID: 31481446 Free PMC article.
-
Induction of metabolism and transport in human intestine: validation of precision-cut slices as a tool to study induction of drug metabolism in human intestine in vitro.Drug Metab Dispos. 2008 Mar;36(3):604-13. doi: 10.1124/dmd.107.018820. Epub 2007 Dec 19. Drug Metab Dispos. 2008. PMID: 18094037
-
Regulation of drug and bile salt transporters in liver and intestine.Drug Metab Rev. 2003 Nov;35(4):305-17. doi: 10.1081/dmr-120026398. Drug Metab Rev. 2003. PMID: 14705863 Review.
-
Impact of Genetic Polymorphisms of ABCB1 (MDR1, P-Glycoprotein) on Drug Disposition and Potential Clinical Implications: Update of the Literature.Clin Pharmacokinet. 2015 Jul;54(7):709-35. doi: 10.1007/s40262-015-0267-1. Clin Pharmacokinet. 2015. PMID: 25860377 Review.
Cited by
-
Knockout of ABC transporters by CRISPR/Cas9 contributes to reliable and accurate transporter substrate identification for drug discovery.Front Pharmacol. 2022 Oct 28;13:1015940. doi: 10.3389/fphar.2022.1015940. eCollection 2022. Front Pharmacol. 2022. PMID: 36386127 Free PMC article.
-
Effect of Genetic Variations in Drug-Metabolizing Enzymes and Drug Transporters on the Pharmacokinetics of Rifamycins: A Systematic Review.Pharmgenomics Pers Med. 2022 Jun 4;15:561-571. doi: 10.2147/PGPM.S363058. eCollection 2022. Pharmgenomics Pers Med. 2022. PMID: 35693129 Free PMC article. Review.
-
Evaluation of the Potency of Anti-HIV and Anti-HCV Drugs to Inhibit P-Glycoprotein Mediated Efflux of Digoxin in Caco-2 Cell Line and Human Precision-Cut Intestinal Slices.Pharmaceuticals (Basel). 2022 Feb 18;15(2):242. doi: 10.3390/ph15020242. Pharmaceuticals (Basel). 2022. PMID: 35215354 Free PMC article.
-
Structural Design and Synthesis of Novel Cyclic Peptide Inhibitors Targeting Mycobacterium tuberculosis Transcription.Life (Basel). 2022 Aug 28;12(9):1333. doi: 10.3390/life12091333. Life (Basel). 2022. PMID: 36143370 Free PMC article.
-
Precision cut intestinal slices, a novel model of acute food allergic reactions.Allergy. 2023 Feb;78(2):500-511. doi: 10.1111/all.15579. Epub 2022 Nov 23. Allergy. 2023. PMID: 36377289 Free PMC article.
References
-
- Albermann N., Schmitz-Winnenthal F. H., Z’graggen K., Volk C., Hoffmann M. M., Haefeli W. E., et al. (2005). Expression of the Drug Transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in Peripheral Blood Mononuclear Cells and Their Relationship with the Expression in Intestine and Liver. Biochem. Pharmacol. 70, 949–958. 10.1016/j.bcp.2005.06.018 - DOI - PubMed
-
- Burger H., Van Tol H., Brok M., Wiemer E. A. C., De Bruijn E. A., Guetens G., et al. (2005). Chronic Imatinib Mesylate Exposure Leads to Reduced Intracellular Drug Accumulation by Induction of the ABCG2 (BCRP) and ABCB1 (MDR1) Drug Transport Pumps. Cancer Biol. Ther. 4, 747–752. 10.4161/cbt.4.7.1826 - DOI - PubMed
LinkOut - more resources
Full Text Sources

