Functional Hyperconnectivity and Task-Based Activity Changes Associated With Neuropathic Pain After Spinal Cord Injury: A Pilot Study
- PMID: 34177753
- PMCID: PMC8222514
- DOI: 10.3389/fneur.2021.613630
Functional Hyperconnectivity and Task-Based Activity Changes Associated With Neuropathic Pain After Spinal Cord Injury: A Pilot Study
Abstract
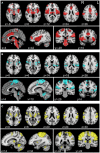
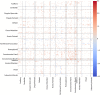
Neuropathic pain (NP) is a devastating chronic pain condition affecting roughly 80% of the spinal cord injury (SCI) patient population. Current treatment options are largely ineffective and neurophysiological mechanisms of NP are not well-understood. Recent studies in neuroimaging have suggested that NP patients have differential patterns of functional activity that are dependent upon the neurological condition causing NP. We conducted an exploratory pilot study to examine functional activation and connectivity in SCI patients with chronic NP compared to SCI patients without NP. We developed a novel somatosensory attention task to identify short term fluctuations in neural activity related to NP vs. non-painful somatosensation using functional magnetic resonance imaging (fMRI). We also collected high-resolution resting state fMRI to identify connectivity-based correlations over time between the two groups. We observed increased activation during focus on NP in brain regions associated with somatosensory integration and representational knowledge in pain subjects when compared with controls. Similarly, NP subjects showed increased connectivity at rest in many of the same areas of the brain, with positive correlations between somatomotor networks, the dorsal attention network, and regions associated with pain and specific areas of painful and non-painful sensation within our cohort. Although this pilot analysis did not identify statistically significant differences between groups after correction for multiple comparisons, the observed correlations between NP and functional activation and connectivity align with a priori hypotheses regarding pain, and provide a well-controlled preliminary basis for future research in this severely understudied patient population. Altogether, this study presents a novel task, identifies regions of increased task-based activation associated with NP after SCI in the insula, prefrontal, and medial inferior parietal cortices, and identifies similar regions of increased functional connectivity associated with NP after SCI in sensorimotor, cingulate, prefrontal, and inferior medial parietal cortices. This, along with our complementary results from a structurally based analysis, provide multi-modal evidence for regions of the brain specific to the SCI cohort as novel areas for further study and potential therapeutic targeting to improve outcomes for NP patients.
Keywords: fMRI; functional connectivity; functional magnetic resonance imaging; neuropathic pain; resting-state fMRI; spinal cord injury; task-based fMRI.
Copyright © 2021 Black, King, Mahan, Anderson and Butson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Center NSCIS . Spinal Cord Injury Facts and Figures at a Glance 2020 SCI Data Sheet. (2020). Available online at: www.msktc.org/sci/model-system-centers (accessed July 2, 2020).
-
- Finnerup NB, Baastrup C, Jensen TS. Neuropathic pain following spinal cord injury pain: mechanisms and treatment. Scand J Pain. (2009) 1(Suppl. 1):S3–11. 10.1016/S1877-8860(09)70003-5 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

