Selective Reporting of Outcomes in Tinnitus Trials: Comparison of Trial Registries With Corresponding Publications
- PMID: 34177776
- PMCID: PMC8222810
- DOI: 10.3389/fneur.2021.669501
Selective Reporting of Outcomes in Tinnitus Trials: Comparison of Trial Registries With Corresponding Publications
Abstract
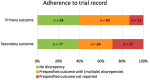
Objectives: We aimed to study the prevalence of selective reporting of primary and secondary outcomes in tinnitus trials and to examine if selective reporting of outcome measures is influenced by the nature and direction of its results. Background: Selective reporting of outcome measures has been reported in several biomedical fields and can influence the clinical usefulness and implementation of outcomes of clinical trials. It is reported as one of the obstacles in finding an effective intervention for tinnitus. Methods: ClinicalTrials.gov (CT.gov) was used to identify all registered interventional tinnitus trials up to December 2015. A standardized search was used to find corresponding publications up to March 2018. The prespecified outcomes in CT.gov were compared with the outcomes reported in corresponding publication(s). The effects of the (lack of) statistical significance of trial results and the effects of funding source on record adherence were evaluated. Changes in registration elements were assessed with the Archive site of CT.gov. Results: We found corresponding publications for 60 (64.5%) of 93 eligible tinnitus trials registered in CT.gov. Of all the publications, five (7.5%) fully reported outcome measures entirely in line with the prespecified outcome measures. Discrepancies between the prespecified and reported outcomes were found in a total of 51 (76.1%) of the studies for primary outcomes, whereas 62 (92.5%) of the studies had discrepancies in secondary outcomes. In secondary outcomes, statistical significance of trial results influenced CT.gov record adherence. In addition, there was a statistically significant difference in the rate of discrepancy in industry-funded [n = 98 (87.5%) discrepant outcomes] and non-industry funded trials [n = 172 (74.5%) discrepant outcomes] (p = 0.01). Finally, 15 (25.9%) trialists made modifications in registered outcome measures during or after the trial period. Conclusion: Tinnitus trials suffer from substantial outcome reporting bias. Awareness of its presence must be raised to limit the obstacles of finding an effective intervention for tinnitus.
Keywords: bias; outcomes; reporting academic misconduct; tinnitus; trials.
Copyright © 2021 Beurden, Beek, Heteren, Smit and Stegeman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Statistical controversies in clinical research: comparison of primary outcomes in protocols, public clinical-trial registries and publications: the example of oncology trials.Ann Oncol. 2017 Apr 1;28(4):688-695. doi: 10.1093/annonc/mdw682. Ann Oncol. 2017. PMID: 28011448
-
Outcome Reporting Bias in Clinical Trials Researching Disease-Modifying Therapy in Patients With Multiple Sclerosis.Neurology. 2024 Mar 26;102(6):e208032. doi: 10.1212/WNL.0000000000208032. Epub 2024 Feb 26. Neurology. 2024. PMID: 38408286
-
Registry versus publication: discrepancy of primary outcomes and possible outcome reporting bias in child and adolescent mental health.Eur Child Adolesc Psychiatry. 2022 May;31(5):757-769. doi: 10.1007/s00787-020-01710-5. Epub 2021 Jan 18. Eur Child Adolesc Psychiatry. 2022. PMID: 33459886
-
Comparison between publicly accessible publications, registries, and protocols of phase III trials indicated persistence of selective outcome reporting.J Clin Epidemiol. 2017 Nov;91:87-94. doi: 10.1016/j.jclinepi.2017.07.010. Epub 2017 Jul 27. J Clin Epidemiol. 2017. PMID: 28757260 Review.
-
Selective outcome reporting in randomized clinical trials of dental implants.J Clin Periodontol. 2019 Jul;46(7):758-765. doi: 10.1111/jcpe.13128. J Clin Periodontol. 2019. PMID: 31077411 Review.
Cited by
-
Discrepancies between pre-specified and reported primary outcomes: A cross-sectional analysis of randomized controlled trials in gastroenterology and hepatology journals.PLoS One. 2024 Nov 22;19(11):e0305027. doi: 10.1371/journal.pone.0305027. eCollection 2024. PLoS One. 2024. PMID: 39576822 Free PMC article.
-
Time to publication for results of clinical trials.Cochrane Database Syst Rev. 2024 Nov 27;11(11):MR000011. doi: 10.1002/14651858.MR000011.pub3. Cochrane Database Syst Rev. 2024. PMID: 39601300
References
Publication types
LinkOut - more resources
Full Text Sources

