2-Heptylcyclopropane-1-Carboxylic Acid Disperses and Inhibits Bacterial Biofilms
- PMID: 34177826
- PMCID: PMC8221421
- DOI: 10.3389/fmicb.2021.645180
2-Heptylcyclopropane-1-Carboxylic Acid Disperses and Inhibits Bacterial Biofilms
Abstract
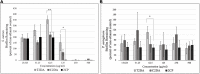
Fatty-acid signaling molecules can inhibit biofilm formation, signal dispersal events, and revert dormant cells within biofilms to a metabolically active state. We synthesized 2-heptylcyclopropane-1-carboxylic acid (2CP), an analog of cis-2-decenoic acid (C2DA), which contains a cyclopropanated bond that may lock the signaling factor in an active state and prevent isomerization to its least active trans-configuration (T2DA). 2CP was compared to C2DA and T2DA for ability to disperse biofilms formed by Staphylococcus aureus and Pseudomonas aeruginosa. 2CP at 125 μg/ml dispersed approximately 100% of S. aureus cells compared to 25% for C2DA; both 2CP and C2DA had significantly less S. aureus biofilm remaining compared to T2DA, which achieved no significant dispersal. 2CP at 125 μg/ml dispersed approximately 60% of P. aeruginosa biofilms, whereas C2DA and T2DA at the same concentration dispersed 40%. When combined with antibiotics tobramycin, tetracycline, or levofloxacin, 2CP decreased the minimum concentration required for biofilm inhibition and eradication, demonstrating synergistic and additive responses for certain combinations. Furthermore, 2CP supported fibroblast viability above 80% for concentrations below 1 mg/ml. This study demonstrates that 2CP shows similar or improved efficacy in biofilm dispersion, inhibition, and eradication compared to C2DA and T2DA and thus may be promising for use in preventing infection for healthcare applications.
Keywords: 2-decenoic acid; Pseudomonas aeruginosa; Staphylococcus aureus; anti-biofilm agents; biofilm; diffusible signaling factors; dispersal.
Copyright © 2021 Harrison, Awais, Harris, Raji, Hoffman, Baker and Jennings.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

