Porcine RIG-I and MDA5 Signaling CARD Domains Exert Similar Antiviral Function Against Different Viruses
- PMID: 34177861
- PMCID: PMC8226225
- DOI: 10.3389/fmicb.2021.677634
Porcine RIG-I and MDA5 Signaling CARD Domains Exert Similar Antiviral Function Against Different Viruses
Abstract
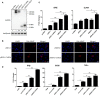
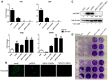
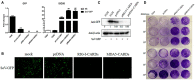
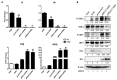
The RIG-I-like receptors (RLRs) RIG-I and MDA5 play critical roles in sensing and fighting viral infections. Although RIG-I and MDA5 have similar molecular structures, these two receptors have distinct features during activation. Further, the signaling domains of the N terminal CARD domains (CARDs) in RIG-I and MDA5 share poor similarity. Therefore, we wonder whether the CARDs of RIG-I and MDA5 play similar roles in signaling and antiviral function. Here we expressed porcine RIG-I and MDA5 CARDs in 293T cells and porcine alveolar macrophages and found that MDA5 CARDs exhibit higher expression and stronger signaling activity than RIG-I CARDs. Nevertheless, both RIG-I and MDA5 CARDs exert comparable antiviral function against several viruses. Transcriptome analysis showed that MDA5 CARDs are more effective in regulating downstream genes. However, in the presence of virus, both RIG-I and MDA5 CARDs exhibit similar effects on downstream gene transcriptions, reflecting their antiviral function.
Keywords: CARD; RLRs; antiviral function; porcine; signaling activity; transcriptome.
Copyright © 2021 Li, Shao, Zhu, Ji, Luo, Xu, Liu, Zheng, Chen, Meurens and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Analysis of Porcine RIG-I Like Receptors Revealed the Positive Regulation of RIG-I and MDA5 by LGP2.Front Immunol. 2021 May 18;12:609543. doi: 10.3389/fimmu.2021.609543. eCollection 2021. Front Immunol. 2021. PMID: 34093517 Free PMC article.
-
Evolution of a Novel Antiviral Immune-Signaling Interaction by Partial-Gene Duplication.PLoS One. 2015 Sep 10;10(9):e0137276. doi: 10.1371/journal.pone.0137276. eCollection 2015. PLoS One. 2015. PMID: 26356745 Free PMC article.
-
Accessory Factors of Cytoplasmic Viral RNA Sensors Required for Antiviral Innate Immune Response.Front Immunol. 2016 May 25;7:200. doi: 10.3389/fimmu.2016.00200. eCollection 2016. Front Immunol. 2016. PMID: 27252702 Free PMC article. Review.
-
Human Hemoglobin Subunit Beta Functions as a Pleiotropic Regulator of RIG-I/MDA5-Mediated Antiviral Innate Immune Responses.J Virol. 2019 Jul 30;93(16):e00718-19. doi: 10.1128/JVI.00718-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31167908 Free PMC article.
-
Structures of RIG-I-Like Receptors and Insights into Viral RNA Sensing.Adv Exp Med Biol. 2019;1172:157-188. doi: 10.1007/978-981-13-9367-9_8. Adv Exp Med Biol. 2019. PMID: 31628656 Review.
Cited by
-
Intestinal-pulmonary axis: a 'Force For Good' against respiratory viral infections.Front Immunol. 2025 Mar 18;16:1534241. doi: 10.3389/fimmu.2025.1534241. eCollection 2025. Front Immunol. 2025. PMID: 40170840 Free PMC article. Review.
-
Rotavirus Infection in Swine: Genotypic Diversity, Immune Responses, and Role of Gut Microbiome in Rotavirus Immunity.Pathogens. 2022 Sep 22;11(10):1078. doi: 10.3390/pathogens11101078. Pathogens. 2022. PMID: 36297136 Free PMC article. Review.
-
Development of Specific Monoclonal Antibodies against Porcine RIG-I-like Receptors Revealed the Species Specificity.Int J Mol Sci. 2023 Feb 18;24(4):4118. doi: 10.3390/ijms24044118. Int J Mol Sci. 2023. PMID: 36835527 Free PMC article.
-
RIG-I-like Receptor Regulation of Immune Cell Function and Therapeutic Implications.J Immunol. 2022 Sep 1;209(5):845-854. doi: 10.4049/jimmunol.2200395. J Immunol. 2022. PMID: 36130131 Free PMC article. Review.
-
Positive Selection Drives the Adaptive Evolution of Mitochondrial Antiviral Signaling (MAVS) Proteins-Mediating Innate Immunity in Mammals.Front Vet Sci. 2022 Jan 31;8:814765. doi: 10.3389/fvets.2021.814765. eCollection 2021. Front Vet Sci. 2022. PMID: 35174241 Free PMC article.
References
-
- Dong X. Y., Liu W. J., Zhao M. Q., Wang J. Y., Pei J. J., Luo Y. W., et al. (2013). Classical swine fever virus triggers RIG-I and MDA5-dependent signaling pathway to IRF-3 and NF-kappaB activation to promote secretion of interferon and inflammatory cytokines in porcine alveolar macrophages. Virol. J. 10:286. 10.1186/1743-422x-10-286 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

