Inflammasomes and Fibrosis
- PMID: 34177893
- PMCID: PMC8226128
- DOI: 10.3389/fimmu.2021.643149
Inflammasomes and Fibrosis
Abstract
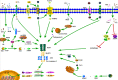
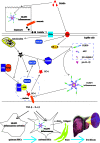
Fibrosis is the final common pathway of inflammatory diseases in various organs. The inflammasomes play an important role in the progression of fibrosis as innate immune receptors. There are four main members of the inflammasomes, such as NOD-like receptor protein 1 (NLRP1), NOD-like receptor protein 3 (NLRP3), NOD-like receptor C4 (NLRC4), and absent in melanoma 2 (AIM2), among which NLRP3 inflammasome is the most studied. NLRP3 inflammasome is typically composed of NLRP3, ASC and pro-caspase-1. The activation of inflammasome involves both "classical" and "non-classical" pathways and the former pathway is better understood. The "classical" activation pathway of inflammasome is that the backbone protein is activated by endogenous/exogenous stimulation, leading to inflammasome assembly. After the formation of "classic" inflammasome, pro-caspase-1 could self-activate. Caspase-1 cleaves cytokine precursors into mature cytokines, which are secreted extracellularly. At present, the "non-classical" activation pathway of inflammasome has not formed a unified model for activation process. This article reviews the role of NLRP1, NLRP3, NLRC4, AIM2 inflammasome, Caspase-1, IL-1β, IL-18 and IL-33 in the fibrogenesis.
Keywords: IL-1β; NLRP3; caspase-1; fibrosis; inflammasome.
Copyright © 2021 Zhang, Chen, Zhou, Wu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis.Front Immunol. 2018 Jun 4;9:1197. doi: 10.3389/fimmu.2018.01197. eCollection 2018. Front Immunol. 2018. PMID: 29915579 Free PMC article.
-
Critical role for the AIM2 inflammasome during acute CNS bacterial infection.J Neurochem. 2014 May;129(4):704-11. doi: 10.1111/jnc.12669. Epub 2014 Feb 19. J Neurochem. 2014. PMID: 24484406 Free PMC article.
-
An optimized whole blood assay measuring expression and activity of NLRP3, NLRC4 and AIM2 inflammasomes.Clin Immunol. 2018 Jun;191:100-109. doi: 10.1016/j.clim.2017.11.011. Epub 2017 Nov 26. Clin Immunol. 2018. PMID: 29183866
-
Novel aspects of the assembly and activation of inflammasomes with focus on the NLRC4 inflammasome.Int Immunol. 2018 Apr 25;30(5):183-193. doi: 10.1093/intimm/dxy009. Int Immunol. 2018. PMID: 29617808 Free PMC article. Review.
-
["The inflammasomes"].Nihon Rinsho Meneki Gakkai Kaishi. 2011;34(5):346-54. doi: 10.2177/jsci.34.346. Nihon Rinsho Meneki Gakkai Kaishi. 2011. PMID: 22041421 Review. Japanese.
Cited by
-
Aucubin suppresses TLR4/NF-κB signalling to shift macrophages toward M2 phenotype in glucocorticoid-associated osteonecrosis of the femoral head.J Cell Mol Med. 2024 Aug;28(15):e18583. doi: 10.1111/jcmm.18583. J Cell Mol Med. 2024. PMID: 39123292 Free PMC article.
-
Rhubarb free anthraquinones improved mice nonalcoholic fatty liver disease by inhibiting NLRP3 inflammasome.J Transl Med. 2022 Jun 28;20(1):294. doi: 10.1186/s12967-022-03495-4. J Transl Med. 2022. PMID: 35765026 Free PMC article.
-
Chinese Herbal Medicine Suyin Detoxification Granule Inhibits Pyroptosis and Epithelial-Mesenchymal Transition by Downregulating MAVS/NLRP3 to Alleviate Renal Injury.J Inflamm Res. 2021 Dec 7;14:6601-6618. doi: 10.2147/JIR.S341598. eCollection 2021. J Inflamm Res. 2021. PMID: 34908861 Free PMC article.
-
NLRP3 Inflammasome in Acute and Chronic Liver Diseases.Int J Mol Sci. 2024 Apr 20;25(8):4537. doi: 10.3390/ijms25084537. Int J Mol Sci. 2024. PMID: 38674122 Free PMC article. Review.
-
New insights into SUMOylation and NEDDylation in fibrosis.Front Pharmacol. 2024 Dec 4;15:1476699. doi: 10.3389/fphar.2024.1476699. eCollection 2024. Front Pharmacol. 2024. PMID: 39697538 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

