Bovine Delta Papillomavirus E5 Oncoprotein Interacts With TRIM25 and Hampers Antiviral Innate Immune Response Mediated by RIG-I-Like Receptors
- PMID: 34177899
- PMCID: PMC8223750
- DOI: 10.3389/fimmu.2021.658762
Bovine Delta Papillomavirus E5 Oncoprotein Interacts With TRIM25 and Hampers Antiviral Innate Immune Response Mediated by RIG-I-Like Receptors
Abstract
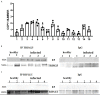
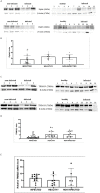
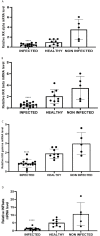
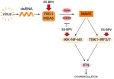
Persistent infection and tumourigenesis by papillomaviruses (PVs) require viral manipulation of various of cellular processes, including those involved in innate immune responses. Herein, we showed that bovine PV (BPV) E5 oncoprotein interacts with a tripartite motif-containing 25 (TRIM25) but not with Riplet in spontaneous BPV infection of urothelial cells of cattle. Statistically significant reduced protein levels of TRIM25, retinoic acid-inducible gene I (RIG-I), and melanoma differentiation-associated gene 5 (MDA5) were detected by Western blot analysis. Real-time quantitative PCR revealed marked transcriptional downregulation of RIG-I and MDA5 in E5-expressing cells compared with healthy urothelial cells. Mitochondrial antiviral signalling (MAVS) protein expression did not vary significantly between diseased and healthy cells. Co-immunoprecipitation studies showed that MAVS interacted with a protein network composed of Sec13, which is a positive regulator of MAVS-mediated RLR antiviral signalling, phosphorylated TANK binding kinase 1 (TBK1), and phosphorylated interferon regulatory factor 3 (IRF3). Immunoblotting revealed significantly low expression levels of Sec13 in BPV-infected cells. Low levels of Sec13 resulted in a weaker host antiviral immune response, as it attenuates MAVS-mediated IRF3 activation. Furthermore, western blot analysis revealed significantly reduced expression levels of pTBK1, which plays an essential role in the activation and phosphorylation of IRF3, a prerequisite for the latter to enter the nucleus to activate type 1 IFN genes. Our results suggested that the innate immune signalling pathway mediated by RIG-I-like receptors (RLRs) was impaired in cells infected with BPVs. Therefore, an effective immune response is not elicited against these viruses, which facilitates persistent viral infection.
Keywords: bovine papilloma virus E5 oncoprotein; melanoma differentiation-associated gene 5 (MDA5); mitochondrial antiviral-signalling (MAVS) protein; retinoic acid-inducible gene I (RIG-I); tripartite motif containing 25 (TRIM25).
Copyright © 2021 De Falco, Cutarelli, Gentile, Cerino, Uleri, Catoi and Roperto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Bovine delta papillomavirus E5 oncoprotein negatively regulates the cGAS-STING signaling pathway in cattle in a spontaneous model of viral disease.Front Immunol. 2022 Oct 12;13:937736. doi: 10.3389/fimmu.2022.937736. eCollection 2022. Front Immunol. 2022. PMID: 36311756 Free PMC article.
-
Subcellular Localizations of RIG-I, TRIM25, and MAVS Complexes.J Virol. 2017 Jan 3;91(2):e01155-16. doi: 10.1128/JVI.01155-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27807226 Free PMC article.
-
The Human Papillomavirus E6 Oncoprotein Targets USP15 and TRIM25 To Suppress RIG-I-Mediated Innate Immune Signaling.J Virol. 2018 Feb 26;92(6):e01737-17. doi: 10.1128/JVI.01737-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29263274 Free PMC article.
-
Recent Advances and Contradictions in the Study of the Individual Roles of Ubiquitin Ligases That Regulate RIG-I-Like Receptor-Mediated Antiviral Innate Immune Responses.Front Immunol. 2020 Jun 24;11:1296. doi: 10.3389/fimmu.2020.01296. eCollection 2020. Front Immunol. 2020. PMID: 32670286 Free PMC article. Review.
-
Negative regulators of the RIG-I-like receptor signaling pathway.Eur J Immunol. 2017 Apr;47(4):615-628. doi: 10.1002/eji.201646484. Eur J Immunol. 2017. PMID: 28295214 Free PMC article. Review.
Cited by
-
Pteridium spp. and Bovine Papillomavirus: Partners in Cancer.Front Vet Sci. 2021 Nov 2;8:758720. doi: 10.3389/fvets.2021.758720. eCollection 2021. Front Vet Sci. 2021. PMID: 34796228 Free PMC article. Review.
-
Immunotherapy of Equine Sarcoids-From Early Approaches to Innovative Vaccines.Vaccines (Basel). 2023 Mar 30;11(4):769. doi: 10.3390/vaccines11040769. Vaccines (Basel). 2023. PMID: 37112681 Free PMC article. Review.
-
Bovine delta papillomavirus E5 oncoprotein negatively regulates the cGAS-STING signaling pathway in cattle in a spontaneous model of viral disease.Front Immunol. 2022 Oct 12;13:937736. doi: 10.3389/fimmu.2022.937736. eCollection 2022. Front Immunol. 2022. PMID: 36311756 Free PMC article.
-
Molecular findings and virological assessment of bladder papillomavirus infection in cattle.Vet Q. 2024 Dec;44(1):1-7. doi: 10.1080/01652176.2024.2387072. Epub 2024 Aug 4. Vet Q. 2024. PMID: 39097798 Free PMC article.
-
Trim47 prevents hematopoietic stem cell exhaustion during stress by regulating MAVS-mediated innate immune pathway.Nat Commun. 2024 Aug 8;15(1):6787. doi: 10.1038/s41467-024-51199-8. Nat Commun. 2024. PMID: 39117713 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

