Blood Cardioplegia Induction, Perfusion Storage and Graft Dysfunction in Cardiac Xenotransplantation
- PMID: 34177906
- PMCID: PMC8220198
- DOI: 10.3389/fimmu.2021.667093
Blood Cardioplegia Induction, Perfusion Storage and Graft Dysfunction in Cardiac Xenotransplantation
Abstract
Background: Perioperative cardiac xenograft dysfunction (PCXD) describes a rapidly developing loss of cardiac function after xenotransplantation. PCXD occurs despite genetic modifications to increase compatibility of the heart. We report on the incidence of PCXD using static preservation in ice slush following crystalloid or blood-based cardioplegia versus continuous cold perfusion with XVIVO© heart solution (XHS) based cardioplegia.
Methods: Baboons were weight matched to genetically engineered swine heart donors. Cardioplegia volume was 30 cc/kg by donor weight, with del Nido cardioplegia and the addition of 25% by volume of donor whole blood. Continuous perfusion was performed using an XVIVO © Perfusion system with XHS to which baboon RBCs were added.
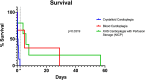
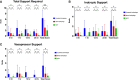
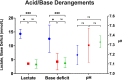
Results: PCXD was observed in 5/8 that were preserved with crystalloid cardioplegia followed by traditional cold, static storage on ice. By comparison, when blood cardioplegia was used followed by cold, static storage, PCXD occurred in 1/3 hearts and only in 1/5 hearts that were induced with XHS blood cardioplegia followed by continuous perfusion. Survival averaged 17 hours in those with traditional preservation and storage, followed by 11.47 days and 15.03 days using blood cardioplegia and XHS+continuous preservation, respectively. Traditional preservation resulted in more inotropic support and higher average peak serum lactate 14.3±1.7 mmol/L compared to blood cardioplegia 3.6±3.0 mmol/L and continuous perfusion 3.5±1.5 mmol/L.
Conclusion: Blood cardioplegia induction, alone or followed by XHS perfusion storage, reduced the incidence of PCXD and improved graft function and survival, relative to traditional crystalloid cardioplegia-slush storage alone.
Keywords: cardiac preservation; cardiac xenotransplantation; graft dysfunction; heart failure; heart transplant; ventricular assist device (VAD); xenotransplantation.
Copyright © 2021 Goerlich, Griffith, Singh, Abdullah, Singireddy, Kolesnik, Lewis, Sentz, Tatarov, Hershfeld, Zhang, Strauss, Odonkor, Williams, Tabatabai, Bhutta, Ayares, Kaczorowski and Mohiuddin.
Conflict of interest statement
DA is employed by Revivicor, Inc., a subsidiary of United Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

