Treg: A Promising Immunotherapeutic Target in Oral Diseases
- PMID: 34177907
- PMCID: PMC8222692
- DOI: 10.3389/fimmu.2021.667862
Treg: A Promising Immunotherapeutic Target in Oral Diseases
Abstract
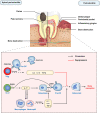
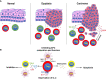
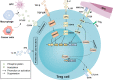
With the pandemic of COVID-19, maintenance of oral health has increasingly become the main challenge of global health. Various common oral diseases, such as periodontitis and oral cancer, are closely associated with immune disorders in the oral mucosa. Regulatory T cells (Treg) are essential for maintaining self-tolerance and immunosuppression. During the process of periodontitis and apical periodontitis, two typical chronic immune-inflammatory diseases, Treg contributes to maintain host immune homeostasis and minimize tissue damage. In contrast, in the development of oral precancerous lesions and oral cancer, Treg is expected to be depleted or down-regulated to enhance the anti-tumor immune response. Therefore, a deeper understanding of the distribution, function, and regulatory mechanisms of Treg cells may provide a prospect for the immunotherapy of oral diseases. In this review, we summarize the distribution and multiple roles of Treg in different oral diseases and discuss the possible mechanisms involved in Treg cell regulation, hope to provide a reference for future Treg-targeted immunotherapy in the treatment of oral diseases.
Keywords: Treg; immunotherapy; oral cancer; oral diseases; periodontitis.
Copyright © 2021 Zhang, Guo and Jia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Regulatory T Lymphocytes in Periodontitis: A Translational View.Mediators Inflamm. 2018 Apr 2;2018:7806912. doi: 10.1155/2018/7806912. eCollection 2018. Mediators Inflamm. 2018. PMID: 29805313 Free PMC article. Review.
-
Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis.Semin Cancer Biol. 2020 Aug;64:29-35. doi: 10.1016/j.semcancer.2019.01.006. Epub 2019 Feb 1. Semin Cancer Biol. 2020. PMID: 30716481 Review.
-
Stabilizing human regulatory T cells for tolerance inducing immunotherapy.Immunotherapy. 2017 Aug;9(9):735-751. doi: 10.2217/imt-2017-0017. Epub 2017 Aug 3. Immunotherapy. 2017. PMID: 28771099 Review.
-
Phenotypes, roles, and modulation of regulatory lymphocytes in periodontitis and its associated systemic diseases.J Leukoc Biol. 2022 Feb;111(2):451-467. doi: 10.1002/JLB.3VMR0321-027RRR. Epub 2021 Apr 22. J Leukoc Biol. 2022. PMID: 33884656 Review.
-
Immunotherapy of Cancer by Targeting Regulatory T cells.Int Immunopharmacol. 2022 Mar;104:108469. doi: 10.1016/j.intimp.2021.108469. Epub 2022 Jan 7. Int Immunopharmacol. 2022. PMID: 35008005 Review.
Cited by
-
Towards multiomic analysis of oral mucosal pathologies.Semin Immunopathol. 2023 Jan;45(1):111-123. doi: 10.1007/s00281-022-00982-0. Epub 2023 Feb 15. Semin Immunopathol. 2023. PMID: 36790488 Free PMC article. Review.
-
Immune Tolerance in the Oral Mucosa.Int J Mol Sci. 2021 Nov 10;22(22):12149. doi: 10.3390/ijms222212149. Int J Mol Sci. 2021. PMID: 34830032 Free PMC article. Review.
-
The niche-specialist and age-related oral microbial ecosystem: crosstalk with host immune cells in homeostasis.Microb Genom. 2022 Jun;8(6):mgen000811. doi: 10.1099/mgen.0.000811. Microb Genom. 2022. PMID: 35731208 Free PMC article.
-
Black raspberry extract inhibits regulatory T-cell activity in a murine model of head and neck squamous cell carcinoma chemoprevention.Front Immunol. 2022 Aug 9;13:932742. doi: 10.3389/fimmu.2022.932742. eCollection 2022. Front Immunol. 2022. PMID: 36016924 Free PMC article.
-
Metabolic targeting of regulatory T cells in oral squamous cell carcinoma: new horizons in immunotherapy.Mol Cancer. 2024 Dec 19;23(1):273. doi: 10.1186/s12943-024-02193-7. Mol Cancer. 2024. PMID: 39696340 Free PMC article. Review.
References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Pillars Article: Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor Alpha-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J Immunol (1995) 155:1151–64. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

