PDCD1 Polymorphisms May Predict Response to Anti-PD-1 Blockade in Patients With Metastatic Melanoma
- PMID: 34177913
- PMCID: PMC8220213
- DOI: 10.3389/fimmu.2021.672521
PDCD1 Polymorphisms May Predict Response to Anti-PD-1 Blockade in Patients With Metastatic Melanoma
Abstract
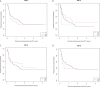
A significant number of patients (pts) with metastatic melanoma do not respond to anti-programmed cell death 1 (PD1) therapies. Identifying predictive biomarkers therefore remains an urgent need. We retrospectively analyzed plasma DNA of pts with advanced melanoma treated with PD-1 antibodies, nivolumab or pembrolizumab, for five PD-1 genotype single nucleotide polymorphisms (SNPs): PD1.1 (rs36084323, G>A), PD1.3 (rs11568821, G>A), PD1.5 (rs2227981, C>T) PD1.6 (rs10204225, G>A) and PD1.9 (rs2227982, C>T). Clinico-pathological and treatment parameters were collected, and presence of SNPs correlated with response, progression free survival (PFS) and overall survival (OS). 115 patients were identified with a median follow up of 18.7 months (range 0.26 - 52.0 months). All were Caucasian; 27% BRAF V600 mutation positive. At PD-1 antibody commencement, 36% were treatment-naïve and 52% had prior ipilimumab. The overall response rate was 43%, 19% achieving a complete response. Overall median PFS was 11.0 months (95% CI 5.4 - 17.3) and median OS was 31.1 months (95% CI 23.2 - NA). Patients with the G/G genotype had more complete responses than with A/G genotype (16.5% vs. 2.6% respectively) and the G allele of PD1.3 rs11568821 was significantly associated with a longer median PFS than the AG allele, 14.1 vs. 7.0 months compared to the A allele (p=0.04; 95% CI 0.14 - 0.94). No significant association between the remaining SNPs and responses, PFS or OS were observed. Despite limitations in sample size, this is the first study to demonstrate an association of a germline PD-1 polymorphism and PFS in response to anti-PD-1 therapy in pts with metastatic melanoma. Extrinsic factors like host germline polymorphisms should be considered with tumor intrinsic factors as predictive biomarkers for immune checkpoint regulators.
Keywords: PD1; immunotherapy; metastatic melanoma; polymorphism; predictive biomarker.
Copyright © 2021 Parakh, Musafer, Paessler, Witkowski, Suen, Tutuka, Carlino, Menzies, Scolyer, Cebon, Dobrovic, Long, Klein and Behren.
Conflict of interest statement
MC has a consultant advisory role with BMS, MSD, Amgen, Novartis, Pierre Fabre, Roche, Sanofi, Merck and Co, Ideaya, Regeneron, Nektar, Eisai and Q biotics and OncoSec. AMM is a consultant advisor to BMS, MSD, Novartis, Roche, Pierre-Fabre, QBiotics. RAS has received fees for professional services from Qbiotics, Novartis, Merck Sharp & Dohme, NeraCare, AMGEN Inc., Bristol-Myers Squibb, Myriad Genetics, and GlaxoSmithKline. JC has sat on advisory boards for Novartis and GSK. GL is consultant advisor for Aduro Biotech Inc, Amgen Inc, Array Biopharma inc, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Hexel AG, Highlight Therapeutics S.L., Merck Sharpe & Dohme, Novartis Pharma AG, OncoSec, Pierre Fabre, QBiotics Group Limited, Regeneron Pharmaceuticals Inc, SkylineDX B.V., and Specialised Therapeutics Australia Pty Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Circulating extracellular vesicles expressing PD1 and PD-L1 predict response and mediate resistance to checkpoint inhibitors immunotherapy in metastatic melanoma.Mol Cancer. 2022 Jan 18;21(1):20. doi: 10.1186/s12943-021-01490-9. Mol Cancer. 2022. PMID: 35042524 Free PMC article.
-
Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study.Lancet Oncol. 2021 Jun;22(6):836-847. doi: 10.1016/S1470-2045(21)00097-8. Epub 2021 May 11. Lancet Oncol. 2021. PMID: 33989557
-
Real-world efficacy of anti-PD-1 antibody or combined anti-PD-1 plus anti-CTLA-4 antibodies, with or without radiotherapy, in advanced mucosal melanoma patients: A retrospective, multicenter study.Eur J Cancer. 2021 Nov;157:361-372. doi: 10.1016/j.ejca.2021.08.034. Epub 2021 Sep 23. Eur J Cancer. 2021. PMID: 34563991
-
Systematic literature review for the association of biomarkers with efficacy of anti-PD-1 inhibitors in advanced melanoma.Future Oncol. 2021 Jul;17(20):2683-2692. doi: 10.2217/fon-2021-0154. Epub 2021 Mar 30. Future Oncol. 2021. PMID: 33783230
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
-
Analysis of Programmed Cell Death-1 (PD-1) Gene Variations (re11568821 and rs41386349) in HTLV-1 Infection Using One Primer Pair and Proviral Load.J Mol Evol. 2023 Aug;91(4):562-566. doi: 10.1007/s00239-023-10104-5. Epub 2023 Apr 5. J Mol Evol. 2023. PMID: 37020064
-
'Know thyself' - host factors influencing cancer response to immune checkpoint inhibitors.J Pathol. 2022 Jul;257(4):513-525. doi: 10.1002/path.5907. Epub 2022 May 20. J Pathol. 2022. PMID: 35394069 Free PMC article. Review.
-
CD274 (PD-L1) Polymorphisms as Predictors of Efficacy in First-Line Platinum-Based Chemotherapy for Extensive-Stage Small Cell Lung Cancer.Int J Mol Sci. 2025 Apr 29;26(9):4245. doi: 10.3390/ijms26094245. Int J Mol Sci. 2025. PMID: 40362483 Free PMC article.
-
Effect of genetic polymorphisms on outcomes following nivolumab for advanced renal cell carcinoma in the SNiP-RCC trial.Cancer Immunol Immunother. 2023 Jun;72(6):1903-1915. doi: 10.1007/s00262-023-03367-w. Epub 2023 Feb 2. Cancer Immunol Immunother. 2023. PMID: 36729213 Free PMC article.
-
Common inherited variants of PDCD1, CD274 and HAVCR2 genes differentially modulate the risk and prognosis of adenocarcinoma and squamous cell carcinoma.J Cancer Res Clin Oncol. 2023 Aug;149(9):6381-6390. doi: 10.1007/s00432-023-04602-8. Epub 2023 Feb 9. J Cancer Res Clin Oncol. 2023. PMID: 36759392 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

