Extracellular Vesicles in Immune System Regulation and Type 1 Diabetes: Cell-to-Cell Communication Mediators, Disease Biomarkers, and Promising Therapeutic Tools
- PMID: 34177928
- PMCID: PMC8219977
- DOI: 10.3389/fimmu.2021.682948
Extracellular Vesicles in Immune System Regulation and Type 1 Diabetes: Cell-to-Cell Communication Mediators, Disease Biomarkers, and Promising Therapeutic Tools
Abstract
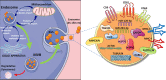
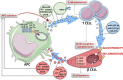
Extracellular vesicles (EVs) are generated by cells of origin through complex molecular mechanisms and released into extracellular environment. Hence, the presence of EVs has been described in multiple biological fluids and in most cases their molecular cargo, which includes non-coding RNAs (ncRNA), messenger RNAs (mRNA), and proteins, has been reported to modulate distinct biological processes. EVs release and their molecular cargo have been demonstrated to be altered in multiple diseases, including autoimmune diseases. Notably, numerous evidence showed a relevant crosstalk between immune system and interacting cells through specific EVs release. The crosstalk between insulin-producing pancreatic β cells and immune system through EVs bidirectional trafficking has yet started to be deciphered, thus uncovering an intricate communication network underlying type 1 diabetes (T1D) pathogenesis. EVs can also be found in blood plasma or serum. Indeed, the assessment of circulating EVs cargo has been shown as a promising advance in the detection of reliable biomarkers of disease progression. Of note, multiple studies showed several specific cargo alterations of EVs collected from plasma/serum of subjects affected by autoimmune diseases, including T1D subjects. In this review, we discuss the recent literature reporting evidence of EVs role in autoimmune diseases, specifically focusing on the bidirectional crosstalk between pancreatic β cells and immune system in T1D and highlight the relevant promising role of circulating EVs as disease biomarkers.
Keywords: autoimmunity; exosomes; extracellular vesicles; immune regulation; pancreatic islets; type 1 diabetes.
Copyright © 2021 Grieco, Fignani, Formichi, Nigi, Licata, Maccora, Brusco, Sebastiani and Dotta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cianciaruso C, Phelps EA, Pasquier M, Hamelin R, Demurtas D, Alibashe Ahmed M, et al. . Primary Human and Rat β-Cells Release the Intracellular Autoantigens GAD65, Ia-2, and Proinsulin in Exosomes Together With Cytokine-Induced Enhancers of Immunity. Diabetes (2017) 66(2):460–73. 10.2337/db16-0671 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

