Venom-Induced Blood Disturbances by Palearctic Viperid Snakes, and Their Relative Neutralization by Antivenoms and Enzyme-Inhibitors
- PMID: 34177943
- PMCID: PMC8222980
- DOI: 10.3389/fimmu.2021.688802
Venom-Induced Blood Disturbances by Palearctic Viperid Snakes, and Their Relative Neutralization by Antivenoms and Enzyme-Inhibitors
Abstract
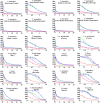
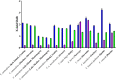
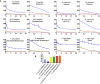
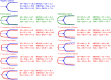
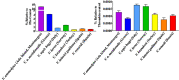
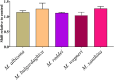
Palearctic vipers are medically significant snakes in the genera Daboia, Macrovipera, Montivipera, and Vipera which occur throughout Europe, Central Asia, Near and Middle East. While the ancestral condition is that of a small-bodied, lowland species, extensive diversification has occurred in body size, and niche specialization. Using 27 venom samples and a panel of in vitro coagulation assays, we evaluated the relative coagulotoxic potency of Palearctic viper venoms and compared their neutralization by three antivenoms (Insoserp Europe, VIPERFAV and ViperaTAb) and two metalloprotease inhibitors (prinomastat and DMPS). We show that variation in morphology parallels variation in the Factor X activating procoagulant toxicity, with the three convergent evolutions of larger body sizes (Daboia genus, Macrovipera genus, and Vipera ammodytes uniquely within the Vipera genus) were each accompanied by a significant increase in procoagulant potency. In contrast, the two convergent evolutions of high altitude specialization (the Montivipera genus and Vipera latastei uniquely within the Vipera genus) were each accompanied by a shift away from procoagulant action, with the Montivipera species being particularly potently anticoagulant. Inoserp Europe and VIPERFAV antivenoms were both effective against a broad range of Vipera species, with Inoserp able to neutralize additional species relative to VIPERFAV, reflective of its more complex antivenom immunization mixture. In contrast, ViperaTAb was extremely potent in neutralizing V. berus but, reflective of this being a monovalent antivenom, it was not effective against other Vipera species. The enzyme inhibitor prinomastat efficiently neutralized the metalloprotease-driven Factor X activation of the procoagulant venoms. In contrast, DMPS (2,3-dimercapto-1-propanesulfonic acid), which as been suggested as another potential treatment option in the absence of antivenom, DMPS failed against all venoms tested. Overall, our results highlight the evolutionary variations within Palearctic vipers and help to inform clinical management of viper envenomation.
Keywords: antivenom; coagulopathy; enzyme inhibition; snakebite; venom.
Copyright © 2021 Chowdhury, Zdenek, Lewin, Carter, Jagar, Ostanek, Harjen, Aldridge, Soria, Haw and Fry.
Conflict of interest statement
ML was employed by the company Ophirex, MA by Micropharm, and RS by Inosan Biopharma, all of which made products tested in this manuscript. However, the companies had no input in experimental design or reviewing of results before publication. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Clinical implications of differential procoagulant toxicity of the palearctic viperid genus Macrovipera, and the relative neutralization efficacy of antivenoms and enzyme inhibitors.Toxicol Lett. 2021 Apr 1;340:77-88. doi: 10.1016/j.toxlet.2020.12.019. Epub 2021 Jan 4. Toxicol Lett. 2021. PMID: 33412251
-
Comparison of Preclinical Properties of Several Available Antivenoms in the Search for Effective Treatment of Vipera ammodytes and Vipera berus Envenoming.Toxins (Basel). 2021 Mar 13;13(3):211. doi: 10.3390/toxins13030211. Toxins (Basel). 2021. PMID: 33805701 Free PMC article.
-
Tiny but Mighty: Vipera ammodytes meridionalis (Eastern Long-Nosed Viper) Ontogenetic Venom Variations in Procoagulant Potency and the Impact on Antivenom Efficacies.Toxins (Basel). 2024 Sep 14;16(9):396. doi: 10.3390/toxins16090396. Toxins (Basel). 2024. PMID: 39330854 Free PMC article.
-
Vipers of Major clinical relevance in Europe: Taxonomy, venom composition, toxicology and clinical management of human bites.Toxicology. 2021 Apr 15;453:152724. doi: 10.1016/j.tox.2021.152724. Epub 2021 Feb 18. Toxicology. 2021. PMID: 33610611 Review.
-
A Guide to the Clinical Management of Vipera Snakebite in Italy.Toxins (Basel). 2024 May 31;16(6):255. doi: 10.3390/toxins16060255. Toxins (Basel). 2024. PMID: 38922149 Free PMC article. Review.
Cited by
-
Considerations for the development of a field-based medical device for the administration of adjunctive therapies for snakebite envenoming.Toxicon X. 2023 Aug 19;20:100169. doi: 10.1016/j.toxcx.2023.100169. eCollection 2023 Dec. Toxicon X. 2023. PMID: 37661997 Free PMC article.
-
Snakebite drug discovery: high-throughput screening to identify novel snake venom metalloproteinase toxin inhibitors.Front Pharmacol. 2024 Jan 11;14:1328950. doi: 10.3389/fphar.2023.1328950. eCollection 2023. Front Pharmacol. 2024. PMID: 38273820 Free PMC article.
-
Venomics and Peptidomics of Palearctic Vipers: A Clade-Wide Analysis of Seven Taxa of the Genera Vipera, Montivipera, Macrovipera, and Daboia across Türkiye.J Proteome Res. 2024 Aug 2;23(8):3524-3541. doi: 10.1021/acs.jproteome.4c00171. Epub 2024 Jul 9. J Proteome Res. 2024. PMID: 38980134 Free PMC article.
-
Extreme Procoagulant Potency in Human Plasma of Venoms from the African Viperid Genera Atheris, Cerastes, and Proatheris and the Relative Efficacy of Antivenoms and Synthetic Enzyme-Inhibitors.Toxins (Basel). 2022 Dec 1;14(12):836. doi: 10.3390/toxins14120836. Toxins (Basel). 2022. PMID: 36548733 Free PMC article.
-
Profiling the Murine Acute Phase and Inflammatory Responses to African Snake Venom: An Approach to Inform Acute Snakebite Pathology.Toxins (Basel). 2022 Mar 22;14(4):229. doi: 10.3390/toxins14040229. Toxins (Basel). 2022. PMID: 35448838 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

