Intramuscular neridronate for the treatment of complex regional pain syndrome type 1: a randomized, double-blind, placebo-controlled study
- PMID: 34178124
- PMCID: PMC8202309
- DOI: 10.1177/1759720X211014020
Intramuscular neridronate for the treatment of complex regional pain syndrome type 1: a randomized, double-blind, placebo-controlled study
Abstract
Background: Complex regional pain syndrome type-1 (CRPS-1) is a severely disabling painful disease challenging to treat. This multicenter, randomized, double-blind placebo-controlled trial examined the efficacy of intramuscular (i.m.) neridronate in CRPS-1 patients.
Methods: A total of 78 patients diagnosed with CRPS-1 (aged 59.5 ± 10.3, 66.7% female) were randomly assigned to 25 mg (i.m.) neridronate (N = 41) given once daily for 16 consecutive days or placebo control (N = 37). Efficacy was assessed after 30 days using a visual analogue scale (VAS) pain score and the number of patients achieving ⩾50% reduction in VAS score. Change in clinical signs and symptoms, quality of life (QoL) using Short Form Health Survey (SF-36) and the McGill Pain Questionnaire were also assessed.
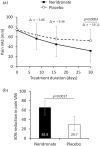
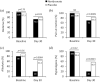
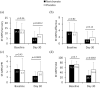
Results: After 30 days, VAS score decreased significantly to a greater extent in neridronate-treated patients versus placebo (31.9 ± 23.3 mm versus 52.3 ± 27.8 mm, p = 0.0003). Furthermore, the proportion of patients achieving a VAS reduction of ⩾50% was greater in the neridronate group (65.9% versus 29.7%, p = 0.0017). Clinical signs and symptoms were improved significantly in the neridronate group versus placebo for edema (72.5% versus 79.9%, p = 0.03), pain during motion (70% versus 83.3%, p = 0.0009), allodynia (20% versus 63.3%, p = 0.0004), and hyperalgesia (20% versus 56.7%, p = 0.0023). Whereas no difference was observed for QoL measures using the SF-36 questionnaire, three of the four pain variables using the McGill Pain Questionnaire improved significantly in the neridronate group. No serious drug-related adverse events were reported during the study.
Conclusion: In patients with acute CRPS-1, i.m. injections of 25 mg neridronate were associated with clinically relevant benefit compared with placebo controls.
Trial registration: EU Clinical Trials Register: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2014-001156-28.
Keywords: algodystrophy; bisphosphonates; complex regional pain syndrome type 1; neridronate; randomized clinical trial.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: Massimo Varenna has received advisory board honoraria from UCB and Kyowa Kirin, consultancy fees from Amgen, and speaker fees from Eli-Lilly and Sandoz. Davide Gatti has received advisory board honoraria from Pfizer, consultancy fees from Abiogen and UCB, and speaker fees from Celgene, Eli-Lilly, and Neopharmed-Gentili. Giovanni Iolascon has received speaker fees from Eli-Lilly and UCB. Maurizio Rossini has received advisory board honoraria and consultancy fees from Abiogen, Celgene, Sanofi, Eli-Lilly, and UCB and speaker fees from Abiogen, Amgen, Abbvie, BMS, Celgene, Grunenthal, Eli-Lilly, MSD, Novartis, Pfizer, Sanofi, Sandoz, and UCB. Fabrizio Nannipieri is an employee of Abiogen Pharma S.p.A. All other authors have no conflict of interest to declare.
Figures
References
-
- Dommerholt J. Complex regional pain syndrome—1: history, diagnostic criteria and etiology. J Bodyw Mov Ther 2004; 8: 167–177.
-
- Bussa M, Guttilla D, Lucia M, et al.. Complex regional pain syndrome type I: a comprehensive review. Acta Anaesthesiol Scand 2015; 59: 685–697. - PubMed
-
- Varenna M, Zucchi F, Ghiringhelli D, et al.. Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol 2000; 27: 1477–1483. - PubMed
LinkOut - more resources
Full Text Sources

